- Record: found
- Abstract: found
- Article: found
Mechanisms by which sialylated milk oligosaccharides impact bone biology in a gnotobiotic mouse model of infant undernutrition
Read this article at
Significance
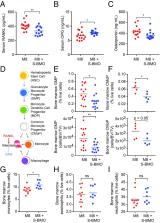
Identifying components of breast milk that influence postnatal development though their effects on the gut microbiota and immune system could provide new therapeutic approaches for childhood undernutrition, including heretofore treatment-refractory linear growth faltering (stunting). Plasma biomarkers of osteoclast-mediated bone resorption and osteoblast-driven bone formation in stunted Bangladeshi children provided evidence for elevated osteoclastic activity. Gnotobiotic mice, colonized with a stunted infant’s gut microbiota, exhibited decreased bone resorption when consuming diets supplemented with a purified bovine oligosaccharide mixture dominated by sialylated structures found in human breast milk. Supplementation decreased osteoclastogenesis while sparing osteoblast activity; the microbiota, intestinal cell populations, and immune mediators contribute to these responses. The influence of milk oligosaccharides on the gut microbiota–bone axis has diagnostic and therapeutic implications.
Abstract
Undernutrition in children is a pressing global health problem, manifested in part by impaired linear growth (stunting). Current nutritional interventions have been largely ineffective in overcoming stunting, emphasizing the need to obtain better understanding of its underlying causes. Treating Bangladeshi children with severe acute malnutrition with therapeutic foods reduced plasma levels of a biomarker of osteoclastic activity without affecting biomarkers of osteoblastic activity or improving their severe stunting. To characterize interactions among the gut microbiota, human milk oligosaccharides (HMOs), and osteoclast and osteoblast biology, young germ-free mice were colonized with cultured bacterial strains from a 6-mo-old stunted infant and fed a diet mimicking that consumed by the donor population. Adding purified bovine sialylated milk oligosaccharides (S-BMO) with structures similar to those in human milk to this diet increased femoral trabecular bone volume and cortical thickness, reduced osteoclasts and their bone marrow progenitors, and altered regulators of osteoclastogenesis and mediators of Th2 responses. Comparisons of germ-free and colonized mice revealed S-BMO-dependent and microbiota-dependent increases in cecal levels of succinate, increased numbers of small intestinal tuft cells, and evidence for activation of a succinate-induced tuft cell signaling pathway linked to Th2 immune responses. A prominent fucosylated HMO, 2′-fucosyllactose, failed to elicit these changes in bone biology, highlighting the structural specificity of the S-BMO effects. These results underscore the need to further characterize the balance between, and determinants of, osteoclastic and osteoblastic activity in stunted infants/children, and suggest that certain milk oligosaccharides may have therapeutic utility in this setting.
Related collections
Most cited references51
- Record: found
- Abstract: found
- Article: found
Maternal and child undernutrition and overweight in low-income and middle-income countries
- Record: found
- Abstract: found
- Article: not found
Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost?
- Record: found
- Abstract: found
- Article: not found