- Record: found
- Abstract: found
- Article: found
Inhibition of the m6A Methyltransferase METTL3 Attenuates the Inflammatory Response in F usarium solani-Induced Keratitis via the NF-κB Signaling Pathway
Read this article at
Abstract
Purpose
The purpose of this study was to elucidate the effect of methyltransferase-like enzyme 3 (METTL3) on inflammation and the NF-κB signaling pathway in fungal keratitis (FK).
Methods
We established corneal stromal cell models and FK mouse models by incubation with Fusarium solani. The overall RNA N6-methyladenosine (m6A) level was determined using an m6A RNA methylation assay kit. The expression of METTL3 was quantified via real-time quantitative polymerase chain reaction (RT–PCR), Western blotting, and immunofluorescence. Subsequently, the level of tumor necrosis factor (TNF) receptor-associated factor 6 (TRAF6) was identified by Western blotting and immunofluorescence. Moreover, we assessed the effect of METTL3 by transfecting cells with siRNA (in vitro) or adeno-associated virus (in vivo). Hematoxylin and eosin (H&E) staining and slit-lamp biomicroscopy were performed to evaluate corneal damage. Furthermore, the state of NF-κB signaling pathway activation was examined by Western blotting. In addition, RT–PCR and enzyme-linked immunosorbent assays (ELISAs) were performed to evaluate levels of the pro-inflammatory factors interleukin-1 β (IL-1 β), interleukin-6 (IL-6) and TNF- ɑ.
Results
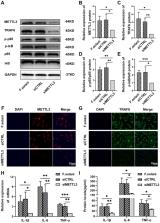
Our data demonstrated that the levels of the RNA m6A methylation and METTL3 were dramatically increased and that the NF-κB signaling pathway was activated in Fusarium solani-induced keratitis. Inhibition of METTL3 decreased the level of TRAF6, downregulated the phospho-p65(p-p65)/p65 and phospho-IκB(p-IκB)/IκB protein ratios, simultaneously attenuating the inflammatory response and fungal burden in FK.
Related collections
Most cited references49
- Record: found
- Abstract: found
- Article: not found
A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation

- Record: found
- Abstract: found
- Article: found
RNA N 6 -methyladenosine methylation in post-transcriptional gene expression regulation

- Record: found
- Abstract: found
- Article: found