- Record: found
- Abstract: found
- Article: found
Protective but Not Anticonvulsant Effects of Ghrelin and JMV-1843 in the Pilocarpine Model of Status epilepticus
Read this article at
Abstract
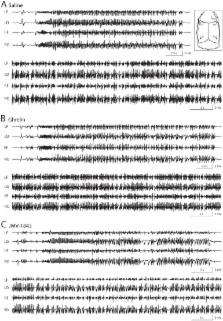
In models of status epilepticus ghrelin displays neuroprotective effects mediated by the growth hormone secretagogue-receptor 1a (GHS-R 1a). This activity may be explained by anticonvulsant properties that, however, are controversial. We further investigated neuroprotection and the effects on seizures by comparing ghrelin with a more effective GHS-R 1a agonist, JMV-1843. Rats were treated either with ghrelin, JMV-1843 or saline 10 min before pilocarpine, which was used to induce status epilepticus. Status epilepticus, developed in all rats, was attenuated by diazepam. No differences were observed among the various groups in the characteristics of pilocarpine-induced seizures. In saline group the area of lesion, characterized by lack of glial fibrillary acidic protein immunoreactivity, was of 0.45±0.07 mm 2 in the hippocampal stratum lacunosum-moleculare, and was accompanied by upregulation of laminin immunostaining, and by increased endothelin-1 expression. Both ghrelin ( P<0.05) and JMV-1843 ( P<0.01) were able to reduce the area of loss in glial fibrillary acidic protein immunostaining. In addition, JMV-1843 counteracted ( P<0.05) the changes in laminin and endothelin-1 expression, both increased in ghrelin-treated rats. JMV-1843 was able to ameliorate neuronal survival in the hilus of dentate gyrus and medial entorhinal cortex layer III ( P<0.05 vs saline and ghrelin groups). These results demonstrate diverse protective effects of growth hormone secretagogues in rats exposed to status epilepticus.
Related collections
Most cited references48
- Record: found
- Abstract: found
- Article: not found
The pilocarpine model of temporal lobe epilepsy
- Record: found
- Abstract: found
- Article: not found
Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration.
- Record: found
- Abstract: found
- Article: not found