- Record: found
- Abstract: found
- Article: found
Claudin-9 constitutes tight junctions of folliculo-stellate cells in the anterior pituitary gland
Read this article at
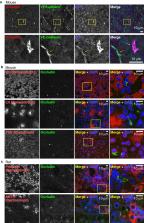
Abstract
The anterior pituitary gland regulates growth, metabolism, and reproduction by secreting hormones. Folliculo-stellate (FS) cells are non-endocrine cells located among hormone-producing cells in the anterior pituitary glands. They form follicular lumens, which are sealed by tight junctions (TJs). Although FS cells are hypothesized to contribute to fine-tuning of endocrine cells, little is known about the exact roles of FS cells. Here, we investigated the molecular composition of TJs in FS cells. We demonstrated that occludin is a good marker for TJs in the pituitary gland and examined the structure of the lumens surrounded by FS cells. We also found that claudin-9 is a major component of TJs in the FS cells. In immunoelectron microscopy, claudin-9 was specifically localized at TJs of the FS cells. The expression of claudin-9 was gradually increased in the pituitary gland after birth, suggesting that claudin-9 is developmentally regulated and performs some specific functions on the paracellular barrier of follicles in the pituitary gland. Furthermore, we found that angulin-1, angulin-2, and tricellulin are localized at the tricellular contacts of the FS cells. Our findings provide a first comprehensive molecular profile of TJs in the FS cells, and may lead us towards unveiling the FS cell functions.
Related collections
Most cited references62
- Record: found
- Abstract: found
- Article: not found
Claudin-1 and -2: Novel Integral Membrane Proteins Localizing at Tight Junctions with No Sequence Similarity to Occludin
- Record: found
- Abstract: found
- Article: not found
Complex phenotype of mice lacking occludin, a component of tight junction strands.
- Record: found
- Abstract: found
- Article: not found