- Record: found
- Abstract: found
- Article: not found
Temporal Evolution of Cortical Ensembles Promoting Remote Memory Retrieval
Read this article at
Abstract
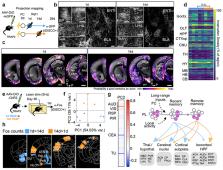
Memories of fearful events can last a lifetime. The prelimbic (PL) subregion of prefrontal cortex plays a critical role in fear memory retrieval over time. Most studies have focused on acquisition, consolidation, and retrieval of recent memories, but much less is known about the neural mechanisms of remote memory. Using a new knock-in mouse for activity-dependent genetic labeling (TRAP2), we demonstrate that neuronal ensembles in PL are dynamic. PL neurons TRAPed during later memory retrievals are more likely to be reactivated and make larger behavioral contributions to remote memory retrieval compared to those TRAPed during learning or early memory retrieval. PL activity during learning is required to initiate this time-dependent reorganization in PL ensembles underlying memory retrieval. Finally, while neurons TRAPed during earlier and later retrievals have similar broad projections throughout the brain, PL neurons TRAPed later have a stronger functional recruitment of cortical targets.
Related collections
Most cited references33
- Record: found
- Abstract: found
- Article: not found
Interplay of hippocampus and prefrontal cortex in memory.
- Record: found
- Abstract: found
- Article: not found
Long-term dynamics of CA1 hippocampal place codes
- Record: found
- Abstract: found
- Article: not found
