- Record: found
- Abstract: found
- Article: found
Discovery proteomics in aging human skeletal muscle finds change in spliceosome, immunity, proteostasis and mitochondria
Read this article at
Abstract
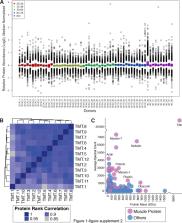
A decline of skeletal muscle strength with aging is a primary cause of mobility loss and frailty in older persons, but the molecular mechanisms of such decline are not understood. Here, we performed quantitative proteomic analysis from skeletal muscle collected from 58 healthy persons aged 20 to 87 years. In muscle from older persons, ribosomal proteins and proteins related to energetic metabolism, including those related to the TCA cycle, mitochondria respiration, and glycolysis, were underrepresented, while proteins implicated in innate and adaptive immunity, proteostasis, and alternative splicing were overrepresented. Consistent with reports in animal models, older human muscle was characterized by deranged energetic metabolism, a pro-inflammatory environment and increased proteolysis. Changes in alternative splicing with aging were confirmed by RNA-seq analysis. We propose that changes in the splicing machinery enables muscle cells to respond to a rise in damage with aging.
eLife digest
As humans age, their muscles become weaker, making it increasingly harder for them to move, a condition known as sarcopenia. Analyzing old muscles in other animals revealed that they produce energy inefficiently, they destroy more proteins than younger muscles, and they have high levels of molecules that cause inflammation. These characteristics may be involved in causing muscle weakness.
Proteomics is the study of proteins, the molecules that play many roles in keeping the body working: for example, they accelerate chemical reactions, participate in copying DNA and help cells respond to stimuli. Using proteomics, it is possible to examine a large number of the different proteins in a tissue, which can provide information about the state of that tissue. Ubaida-Mohien et al. used this approach to answer the question of why muscles become weaker with age.
First, they analyzed the levels of all the proteins found in skeletal muscle collected from 58 healthy volunteers between 20 and 87 years of age. This revealed that the muscles of older people have fewer copies of the proteins that make up ribosomes – the cellular machines that produce new proteins – and fewer proteins involved in providing the cell with chemical energy. In contrast, proteins implicated in the immune system, in the maintenance of existing proteins, and in processing other molecules called RNAs were more abundant in older muscles.
Ubaida-Mohien et al. then looked more closely at changes involving RNA processing. Cells make proteins by copying DNA sequences into an RNA template and using this template to instruct the ribosomes on how to make the specific protein. Before the RNA can be ‘read’ by a ribosome, however, some parts must be cut out and others added, which can lead to different versions of the final RNA, also known as alternative transcripts.
In order to check whether the difference in the levels of proteins that process RNAs was affecting the RNAs being produced, Ubaida-Mohien et al. extracted the RNAs from older and younger muscles and compared them. This showed that the RNA in older people had more alternative transcripts, confirming that the change in protein levels was having downstream effects.
Currently, it is not possible to prevent or delay the loss of muscle strength associated with aging. Understanding how the protein make-up of muscles changes as humans grow older may help find new ways to prevent and perhaps even reverse this decline.
Related collections
Most cited references77
- Record: found
- Abstract: found
- Article: not found
Empirical Statistical Model To Estimate the Accuracy of Peptide Identifications Made by MS/MS and Database Search
- Record: found
- Abstract: found
- Article: not found
Reversed-phase chromatography with multiple fraction concatenation strategy for proteome profiling of human MCF10A cells.
- Record: found
- Abstract: found
- Article: not found