- Record: found
- Abstract: found
- Article: not found
Polyphenol- and fibre-rich dried fruits with green tea attenuate starch-derived postprandial blood glucose and insulin: a randomised, controlled, single-blind, cross-over intervention
Read this article at
Abstract
Polyphenol- and fibre-rich foods (PFRF) have the potential to affect postprandial glycaemic responses by reducing glucose absorption, and thus decreasing the glycaemic response of foods when consumed together. A randomised, single-blind, cross-over study was conducted on sixteen healthy volunteers to test whether PFRF could attenuate postprandial blood glucose in healthy volunteers when added to a source of carbohydrate (starch in bread). This is the first study to examine the effects of a meal comprised of components to inhibit each stage of the biochemical pathway, leading up to the appearance of glucose in the blood. The volunteers were fasted and attended four visits: two control visits (bread, water, balancing sugars) and two test visits (single and double dose of PFRF) where they consumed bread, water and PFRF. Blood samples were collected at 0 (fasted), 15, 30, 45, 60, 90, 120, 150 and 180 min after consumption. The PFRF components were tested for α-amylase and α-glucosidase inhibitory potential in vitro. Plasma glucose was lower after consumption of both doses compared with controls: lower dose, change in mean incremental areas under the glucose curves (IAUC)=−27·4 (sd7·5) %, P<0·001; higher dose, IAUC=−49·0 (sd15·3) %, P<0·001; insulin IAUC was also attenuated by−46·9 (sd13·4) %, P<0·01. Consistent with this, the polyphenol components of the PFRF inhibited α-amylase (green tea, strawberry, blackberry and blackcurrant) and α-glucosidase (green tea) activities in vitro. The PFRF have a pronounced and significant lowering effect on postprandial blood glucose and insulin response in humans, due in part to inhibition of α-amylase and α-glucosidase, as well as glucose transport.
Related collections
Most cited references29
- Record: found
- Abstract: found
- Article: found
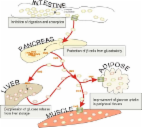
Impact of Dietary Polyphenols on Carbohydrate Metabolism
- Record: found
- Abstract: found
- Article: not found
Low-glycemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials.
- Record: found
- Abstract: found
- Article: not found
