- Record: found
- Abstract: found
- Article: found
Microdomains of muscarinic acetylcholine and Ins(1,4,5) P 3 receptors create ‘Ins(1,4,5) P 3 junctions’ and sites of Ca 2+ wave initiation in smooth muscle
Read this article at
Summary
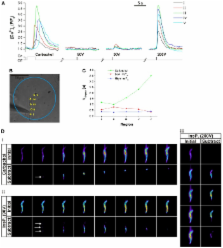
Increases in cytosolic Ca 2+ concentration ([Ca 2+] c) mediated by inositol (1,4,5)-trisphosphate [Ins(1,4,5) P 3, hereafter InsP 3] regulate activities that include division, contraction and cell death. InsP 3-evoked Ca 2+ release often begins at a single site, then regeneratively propagates through the cell as a Ca 2+ wave. The Ca 2+ wave consistently begins at the same site on successive activations. Here, we address the mechanisms that determine the Ca 2+ wave initiation site in intestinal smooth muscle cells. Neither an increased sensitivity of InsP 3 receptors (InsP 3R) to InsP 3 nor regional clustering of muscarinic receptors (mAChR3) or InsP 3R1 explained the selection of an initiation site. However, examination of the overlap of mAChR3 and InsP 3R1 localisation, by centre of mass analysis, revealed that there was a small percentage (∼10%) of sites that showed colocalisation. Indeed, the extent of colocalisation was greatest at the Ca 2+ wave initiation site. The initiation site might arise from a selective delivery of InsP 3 from mAChR3 activity to particular InsP 3Rs to generate faster local [Ca 2+] c increases at sites of colocalisation. In support of this hypothesis, a localised subthreshold ‘priming’ InsP 3 concentration applied rapidly, but at regions distant from the initiation site, shifted the wave to the site of the priming. Conversely, when the Ca 2+ rise at the initiation site was rapidly and selectively attenuated, the Ca 2+ wave again shifted and initiated at a new site. These results indicate that Ca 2+ waves initiate where there is a structural and functional coupling of mAChR3 and InsP 3R1, which generates junctions in which InsP 3 acts as a highly localised signal by being rapidly and selectively delivered to InsP 3R1.
Related collections
Most cited references42
- Record: found
- Abstract: found
- Article: not found
Subtype-specific and ER lumenal environment-dependent regulation of inositol 1,4,5-trisphosphate receptor type 1 by ERp44.
- Record: found
- Abstract: found
- Article: not found
Local and global cytosolic Ca2+ oscillations in exocrine cells evoked by agonists and inositol trisphosphate.
- Record: found
- Abstract: found
- Article: not found