- Record: found
- Abstract: found
- Article: found
Changes in hippocampal AMPA receptors and cognitive impairments in chronic ketamine addiction models: another understanding of ketamine CNS toxicity
Read this article at
Abstract
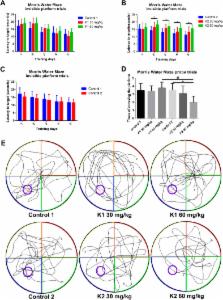
Ketamine has been reported to impair human cognitive function as a recreational drug of abuse. However, chronic effects of ketamine on central nervous system need to be further explored. We set out to establish chronic ketamine addiction models by giving mice a three or six month course of daily intraperitoneal injections of ketamine, then examined whether long-term ketamine administration induced cognition deficits and changed hippocampal post-synaptic protein expression in adult mice. Behavior tests results showed that mice exhibited dose- and time-dependent learning and memory deficits after long-term ketamine administration. Western blot results showed levels of GluA1, p-S845 and p-S831 proteins demonstrated significant decline with ketamine 60 mg/kg until six months administration paradigm. But levels of p-S845 and p-S831 proteins exhibited obvious increase with ketamine 60 mg/kg three months administration paradigm. NR1 protein levels significantly decrease with ketamine 60 mg/kg three and six months administration paradigm. Our results indicate that reduced expression levels and decreased phosphorylation levels of hippocampal post-synaptic membrane GluA1- containing AMPA receptors maybe involved in cognition impairment after long-term ketamine administration. These findings provide further evidence for the cognitive damage of chronic ketamine addiction as a recreational drug.
Related collections
Most cited references44
- Record: found
- Abstract: found
- Article: not found
Learning induces long-term potentiation in the hippocampus.
- Record: found
- Abstract: found
- Article: not found
AMPA receptor trafficking and synaptic plasticity.
- Record: found
- Abstract: found
- Article: not found