- Record: found
- Abstract: found
- Article: found
Intranasal delivery of nanostructured lipid carriers, solid lipid nanoparticles and nanoemulsions: A current overview of in vivo studies
Read this article at
Abstract
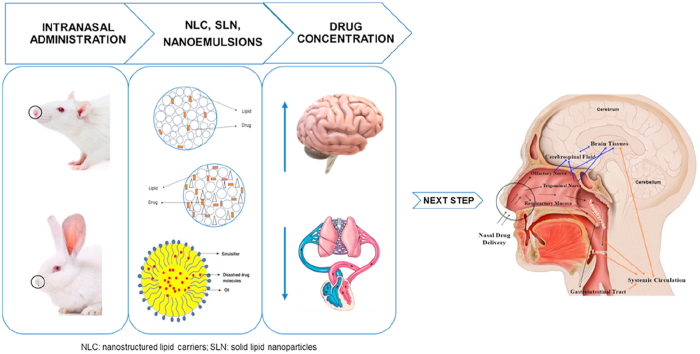
The management of the central nervous system (CNS) disorders is challenging, due to the need of drugs to cross the blood‒brain barrier (BBB) and reach the brain. Among the various strategies that have been studied to circumvent this challenge, the use of the intranasal route to transport drugs from the nose directly to the brain has been showing promising results. In addition, the encapsulation of the drugs in lipid-based nanocarriers, such as solid lipid nanoparticles (SLNs), nanostructured lipid carriers (NLCs) or nanoemulsions (NEs), can improve nose-to-brain transport by increasing the bioavailability and site-specific delivery. This review provides the state-of-the-art of in vivo studies with lipid-based nanocarriers (SLNs, NLCs and NEs) for nose-to-brain delivery. Based on the literature available from the past two years, we present an insight into the different mechanisms that drugs can follow to reach the brain after intranasal administration. The results of pharmacokinetic and pharmacodynamics studies are reported and a critical analysis of the differences between the anatomy of the nasal cavity of the different animal species used in in vivo studies is carried out. Although the exact mechanism of drug transport from the nose to the brain is not fully understood and its effectiveness in humans is unclear, it appears that the intranasal route together with the use of NLCs, SLNs or NEs is advantageous for targeting drugs to the brain. These systems have been shown to be more effective for nose-to-brain delivery than other routes or formulations with non-encapsulated drugs, so they are expected to be approved by regulatory authorities in the coming years.
Graphical abstract
Related collections
Most cited references96

- Record: found
- Abstract: found
- Article: found
Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems
- Record: found
- Abstract: found
- Article: not found
Nanoparticle-mediated brain drug delivery: Overcoming blood-brain barrier to treat neurodegenerative diseases.
- Record: found
- Abstract: found
- Article: not found