- Record: found
- Abstract: found
- Article: not found
Neto1 Is a Novel CUB-Domain NMDA Receptor–Interacting Protein Required for Synaptic Plasticity and Learning
Read this article at
Abstract
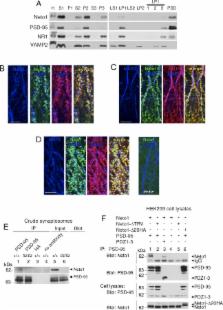
The N-methyl-D-aspartate receptor (NMDAR), a major excitatory ligand-gated ion channel in the central nervous system (CNS), is a principal mediator of synaptic plasticity. Here we report that neuropilin tolloid-like 1 (Neto1), a complement C1r/C1s, Uegf, Bmp1 (CUB) domain-containing transmembrane protein, is a novel component of the NMDAR complex critical for maintaining the abundance of NR2A-containing NMDARs in the postsynaptic density. Neto1-null mice have depressed long-term potentiation (LTP) at Schaffer collateral-CA1 synapses, with the subunit dependency of LTP induction switching from the normal predominance of NR2A- to NR2B-NMDARs. NMDAR-dependent spatial learning and memory is depressed in Neto1-null mice, indicating that Neto1 regulates NMDA receptor-dependent synaptic plasticity and cognition. Remarkably, we also found that the deficits in LTP, learning, and memory in Neto1-null mice were rescued by the ampakine CX546 at doses without effect in wild-type. Together, our results establish the principle that auxiliary proteins are required for the normal abundance of NMDAR subunits at synapses, and demonstrate that an inherited learning defect can be rescued pharmacologically, a finding with therapeutic implications for humans.
Author Summary
The fundamental unit for information processing in the brain is the synapse, a highly specialized site of communication between the brain's multitude of individual neurons. The strength of the communication at each synapse changes in response to neuronal activity—a process called synaptic plasticity—allowing networks of neurons to adapt and learn. How synaptic plasticity occurs is a major question in neurobiology. A central player in synaptic plasticity is an assembly of synaptic proteins called the NMDA receptor complex. Here, we discovered that the protein Neto1 is a component of the NMDA receptor complex. Neto1-deficient mice had a dramatic decrease in the number of NMDA receptors at synapses and consequently, synaptic plasticity and learning were impaired. By indirectly enhancing the function of the residual NMDA receptors in Neto1-deficient mice with a small molecule, we restored synaptic plasticity and learning to normal levels. Our findings establish the principle that inherited abnormalities of synaptic plasticity and learning due to NMDA receptor dysfunction can be pharmacologically corrected. Our discoveries also suggest that synaptic proteins that share a molecular signature, called the CUB domain, with Neto1 may be important components of synaptic receptors across species, because several CUB-domain proteins in worms have also been found to regulate synaptic receptors.
Abstract
Spatial learning and memory depend on the N-methyl-D-aspartic acid receptor, a synaptic ion channel regulated by Neto1. Impaired cognition due to the absence of Neto1 can be rescued pharmacologically, a finding with implications for the therapy of inherited learning defects in humans.
Related collections
Most cited references64
- Record: found
- Abstract: not found
- Article: not found
The glutamate receptor ion channels.
- Record: found
- Abstract: found
- Article: not found
