- Record: found
- Abstract: found
- Article: found
Metabolic Acidosis and Strong Ion Gap in Critically Ill Patients with Acute Kidney Injury
Read this article at
Abstract
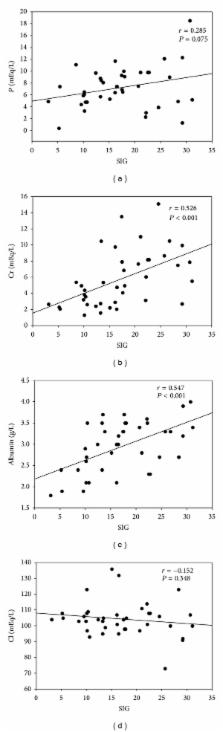
Purpose. To determine the influence of physicochemical parameters on survival in metabolic acidosis (MA) and acute kidney injury (AKI) patients. Materials and Methods. Seventy-eight MA patients were collected and assigned to AKI or non-AKI group. We analyzed the physiochemical parameters on survival at 24 h, 72 h, 1 week, 1 month, and 3 months after AKI. Results. Mortality rate was higher in the AKI group. AKI group had higher anion gap (AG), strong ion gap (SIG), and apparent strong ion difference (SIDa) values than non-AKI group. SIG value was higher in the AKI survivors than nonsurvivors and this value was correlated serum creatinine, phosphate, albumin, and chloride levels. SIG and serum albumin are negatively correlated with Acute Physiology and Chronic Health Evaluation IV scores. AG was associated with mortality at 1 and 3 months post-AKI, whereas SIG value was associated with mortality at 24 h, 72 h, 1 week, 1 month, and 3 months post-AKI. Conclusions. Whether high or low SIG values correlate with mortality in MA patients with AKI depends on its correlation with serum creatinine, chloride, albumin, and phosphate (P) levels. AG predicts short-term mortality and SIG value predicts both short- and long-term mortality among MA patients with AKI.
Related collections
Most cited references23
- Record: found
- Abstract: found
- Article: not found
Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study.
- Record: found
- Abstract: found
- Article: not found
A comparison of three methods to estimate baseline creatinine for RIFLE classification.
- Record: found
- Abstract: found
- Article: not found