- Record: found
- Abstract: found
- Article: not found
Predicting the adult height of girls with central precocious puberty
Read this article at
Summary
Background
There are no absolute criteria for identifying those girls with idiopathic central precocious puberty (CPP) who will benefit from gonadotropin-releasing hormone analog (GnRHa) treatment. Our objective was to predict at initial evaluation the differences between adult height (AH) and target height (TH) and (for untreated girls) the time between puberty onset and first menstruation.
Material/Methods
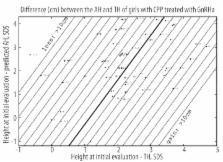
The 122 girls with CPP who reached their AH included 70 who were given GnRHa because their predicted AH was <155 cm (n=24), their luteinising hormone (LH)/follicle-stimulating hormone peaks (FSH) ratio was >0.66 (n=41) and/or their estradiol was >15 pg/ml (n=40). The other 52 were untreated because their predicted AH was >155 cm. Multiple linear regressions were performed on several subsets of variables.
Results
Treated: the difference between AH and TH (−0.6±5.4 cm) was predicted by (using SDS) =3.68 (height at initial evaluation – TH) − 1.94 (height at initial evaluation-predicted AH) − 4.23; R2=0.73. Untreated: the difference between AH and TH (1.7±4.3 cm) was predicted by =2.76 (height at initial evaluation – TH) − 3.68 LH/FSH peaks ratio − 3.49; R2=0.77. Time between puberty onset and first menstruation (years) was predicted by =12.2 – 1.06 age CPP − 0.4 (height at initial evaluation – TH); R2=0.75.
Related collections
Most cited references29
- Record: found
- Abstract: not found
- Article: not found
Standards for children's height at ages 2-9 years allowing for heights of parents.
- Record: found
- Abstract: found
- Article: not found
Body Mass Index variations: centiles from birth to 87 years.
- Record: found
- Abstract: found
- Article: not found
