- Record: found
- Abstract: found
- Article: found
Bone metastases in patients with metastatic renal cell carcinoma: are they always associated with poor prognosis?
Read this article at
Abstract
Purpose
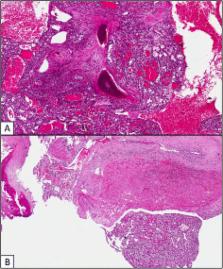
Aim of this study was to investigate for the presence of existing prognostic factors in patients with bone metastases (BMs) from RCC since bone represents an unfavorable site of metastasis for renal cell carcinoma (mRCC).
Materials and methods
Data of patients with BMs from RCC were retrospectively collected. Age, sex, ECOG-Performance Status (PS), MSKCC group, tumor histology, presence of concomitant metastases to other sites, time from nephrectomy to bone metastases (TTBM, classified into three groups: <1 year, between 1 and 5 years and >5 years) and time from BMs to skeletal-related event (SRE) were included in the Cox analysis to investigate their prognostic relevance.
Results
470 patients were enrolled in this analysis. In 19 patients (4%),bone was the only metastatic site; 277 patients had concomitant metastases in other sites. Median time to BMs was 16 months (range 0 − 44y) with Median OS of 17 months. Number of metastatic sites (including bone, p = 0.01), concomitant metastases, high Fuhrman grade (p < 0.001) and non-clear cell histology ( p = 0.013) were significantly associated with poor prognosis. Patients with TTBM >5 years had longer OS (22 months) compared to patients with TTBM <1 year (13 months) or between 1 and 5 years (19 months) from nephrectomy (p < 0.001), no difference was found between these two last groups (p = 0.18). At multivariate analysis, ECOG-PS, MSKCC group and concomitant lung or lymph node metastases were independent predictors of OS in patients with BMs.
Related collections
Most cited references25
- Record: found
- Abstract: found
- Article: not found
Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study.
- Record: found
- Abstract: found
- Article: not found
Interval estimation for the difference between independent proportions: comparison of eleven methods
- Record: found
- Abstract: found
- Article: not found