- Record: found
- Abstract: found
- Article: found
Multistage kinetic analysis of DMAA/MBAM polymer removal from gelcast ceramic parts using a multi-stage parallel reaction model and model-free method
Read this article at
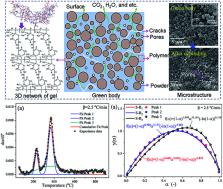
Abstract
This work aims to develop an effective method for investigating the multistage debinding kinetics and reaction mechanisms of removing N, N-dimethylacrylamide/ N, N′-methylenebisacrylamide (DMAA/MBAM) polymer from gelcast ceramic parts. Thermogravimetry (TG) and pyrolysis-gas chromatography/mass spectrometry (Py-GC/MS) experiments were performed to investigate the thermal degradation characteristics and the main compounds produced during the pyrolysis of DMAA/MBAM polymer within green components. A multi-stage parallel reaction model (M-PRM) was proposed to separate the overlapping peaks in the d α/d T curves. The kinetic parameters (activation energy E and pre-exponential factor k 0) of each substage were calculated using model-free methods (Flynn–Wall–Ozawa, Starink, Friedman and Kissinger–Akahira–Sunose) and an activation energy variable model. In addition, the most appropriate kinetic mechanism function f( α) of each substage was analyzed and discussed via Málek's procedure and the Šesták–Berggren (SB) model. The results showed that the DMAA/MBAM polymer burnout in green components can be divided into three substages through a three-stage parallel reaction model (3-PRM). The values of E (Friedman method) for substages 1 to 3 were E( α) = 139.862 − 110.481 α + 156.161 α 2 − 88.714 α 3 kJ mol −1, E( α) = 160.791 + 152.496 α − 236.906 α 2 + 163.724 α 3 kJ mol −1 and E( α) = 72.132 + 452.830 α − 669.039 α 2 + 507.015 α 3 kJ mol −1, respectively. The average values of E showed an increasing tendency from substages 1 to 3, and a kinetic compensation effect was also observed between the E and k 0 in each substage. The kinetic mechanism analysis revealed that the reaction mechanisms for substages 1 to 3 were f( α) = (1 − α) 0.668 α 3.049(−ln(1 − α)) −3.874, f( α) = (1 − α) 0.700 α 3.177(−ln(1 − α)) −3.962 and f( α) = (1 − α) 1.049 α −0.161(−ln(1 − α)) 0.518, respectively. It is expected that the research results can be extended to investigate the multiplex debinding of binders or polymers for various colloidal molding techniques.
Abstract
This work aims to develop an effective method to investigate the multistage debinding kinetics and the reaction mechanisms of removing DMAA/MBAM polymer from gelcast ceramic parts.
