- Record: found
- Abstract: found
- Article: found
High endothelial venule is a surrogate biomarker for T-cell inflamed tumor microenvironment and prognosis in gastric cancer

Read this article at
Abstract
Background
High endothelial venule (HEV) is a specialized vasculature for lymphocyte trafficking. While HEVs are frequently observed within gastric cancer (GC), the vascular–immune interaction between HEV and tumor-infiltrating lymphocytes (TILs) has not been well elucidated. In this study, we aimed to unveil the potential value of HEVs as a surrogate marker for T-cell inflamed immune microenvironment in GC using a large number of prospectively collected surgical specimens of GC.
Methods
We included 460 patients with GC who underwent surgical resection. Nanostring PanCancer immune profiling was performed to evaluate the immunological phenotype of GCs. HEV density and three distinct patterns of TILs (Crohn-like lymphoid reaction, peritumoral lymphoid reaction, and intratumoral lymphoid reaction) were analyzed for their relationship and evaluated as prognostic factors for relapse-free survival (RFS) and overall survival (OS).
Results
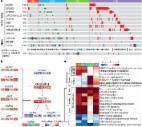
HEV-high GC revealed increased infiltration by immune cell subsets, including dendritic cells, CD8 + cytotoxic T cells, and CD4 + helper T cells. In addition, HEV-high GC demonstrated increased immune-modulating chemokines, type I or II interferon pathway, and immune checkpoints, all of which indicate the inflamed tumor microenvironment (TME). All three distinct patterns of TILs were associated with HEV density. In survival analysis, patients with HEV-high GC displayed significantly longer RFS and OS than those with HEV-low GC (p<0.001 for RFS, p<0.001 for OS). Multivariate analysis demonstrated that HEV was the most significant immunological prognostic factor for RFS (patients with high HEV compared with those with low HEV; HR 0.412, 95% CI 0.241 to 0.705, p=0.001) and OS (HR 0.547, 95% CI 0.329 to 0.909, p=0.02) after adjustment for age, stage, and TIL.
Related collections
Most cited references50
- Record: found
- Abstract: found
- Article: found
Comprehensive molecular characterization of gastric adenocarcinoma
- Record: found
- Abstract: found
- Article: not found
Innate and adaptive immune cells in the tumor microenvironment.
- Record: found
- Abstract: found
- Article: not found