- Record: found
- Abstract: found
- Article: found
Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles and Their Potential as Novel Immunomodulatory Therapeutic Agents
Read this article at
Abstract
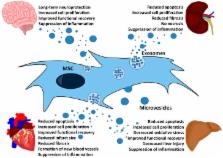
Extracellular vesicles (EVs), such as exosomes and microvesicles, have been identified as mediators of a newly-discovered intercellular communication system. They are essential signaling mediators in various physiological and pathophysiological processes. Depending on their origin, they fulfill different functions. EVs of mesenchymal stem/stromal cells (MSCs) have been found to promote comparable therapeutic activities as MSCs themselves. In a variety of in vivo models, it has been observed that they suppress pro-inflammatory processes and reduce oxidative stress and fibrosis. By switching pro-inflammatory into tolerogenic immune responses, MSC-EVs very likely promote tissue regeneration by creating a pro-regenerative environment allowing endogenous stem and progenitor cells to successfully repair affected tissues. Accordingly, MSC-EVs provide a novel, very promising therapeutic agent, which has already been successfully applied to humans. However, the MSC-EV production process has not been standardized, yet. Indeed, a collection of different protocols has been used for the MSC-EV production, characterization and application. By focusing on kidney, heart, liver and brain injuries, we have reviewed the major outcomes of published MSC-EV in vivo studies.
Related collections
Most cited references35
- Record: found
- Abstract: found
- Article: not found
Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms.
- Record: found
- Abstract: found
- Article: not found
Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo.
- Record: found
- Abstract: found
- Article: not found