- Record: found
- Abstract: found
- Article: found
Vimentin contributes to epithelial-mesenchymal transition cancer cell mechanics by mediating cytoskeletal organization and focal adhesion maturation
Read this article at
Abstract
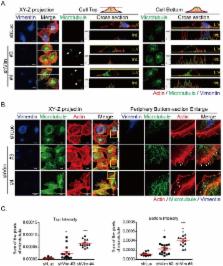
Modulations of cytoskeletal organization and focal adhesion turnover correlate to tumorigenesis and epithelial-mesenchymal transition (EMT), the latter process accompanied by the loss of epithelial markers and the gain of mesenchymal markers (e.g., vimentin). Clinical microarray results demonstrated that increased levels of vimentin mRNA after chemotherapy correlated to a poor prognosis of breast cancer patients. We hypothesized that vimentin mediated the reorganization of cytoskeletons to maintain the mechanical integrity in EMT cancer cells. By using knockdown strategy, the results showed reduced cell proliferation, impaired wound healing, loss of directional migration, and increased large membrane extension in MDA-MB 231 cells. Vimentin depletion also induced reorganization of cytoskeletons and reduced focal adhesions, which resulted in impaired mechanical strength because of reduced cell stiffness and contractile force. In addition, overexpressing vimentin in MCF7 cells increased cell stiffness, elevated cell motility and directional migration, reoriented microtubule polarity, and increased EMT phenotypes due to the increased β1-integrin and the loss of junction protein E-cadherin. The EMT-related transcription factor slug was also mediated by vimentin. The current study demonstrated that vimentin serves as a regulator to maintain intracellular mechanical homeostasis by mediating cytoskeleton architecture and the balance of cell force generation in EMT cancer cells.
Related collections
Most cited references38
- Record: found
- Abstract: found
- Article: not found