- Record: found
- Abstract: found
- Article: found
Predictive Power of In Silico Approach to Evaluate Chemicals against M. tuberculosis: A Systematic Review
Read this article at
Abstract
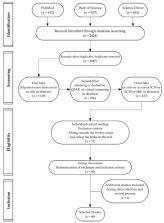
Mycobacterium tuberculosis (Mtb) is an endemic bacterium worldwide that causes tuberculosis (TB) and involves long-term treatment that is not always effective. In this context, several studies are trying to develop and evaluate new substances active against Mtb. In silico techniques are often used to predict the effects on some known target. We used a systematic approach to find and evaluate manuscripts that applied an in silico technique to find antimycobacterial molecules and tried to prove its predictive potential by testing them in vitro or in vivo. After searching three different databases and applying exclusion criteria, we were able to retrieve 46 documents. We found that they all follow a similar screening procedure, but few studies exploited equal targets, exploring the interaction of multiple ligands to 29 distinct enzymes. The following in vitro/vivo analysis showed that, although the virtual assays were able to decrease the number of molecules tested, saving time and money, virtual screening procedures still need to develop the correlation to more favorable in vitro outcomes. We find that the in silico approach has a good predictive power for in vitro results, but call for more studies to evaluate its clinical predictive possibilities.
Related collections
Most cited references77
- Record: found
- Abstract: found
- Article: not found
Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis.

- Record: found
- Abstract: found
- Article: found
Computational methods in drug discovery
- Record: found
- Abstract: found
- Article: not found