- Record: found
- Abstract: found
- Article: found
NADPH Oxidase Hyperactivity Contributes to Cardiac Dysfunction and Apoptosis in Rats with Severe Experimental Pancreatitis through ROS-Mediated MAPK Signaling Pathway
Read this article at
Abstract
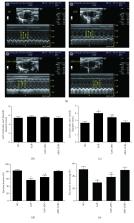
NADPH oxidase (Nox) is considered a major source of reactive oxygen species (ROS) in the heart in normal and pathological conditions. However, the role of Nox in severe acute pancreatitis- (SAP-) associated cardiac injury remains unclear. Therefore, we aim to investigate the contribution of Nox to SAP-associated cardiac injury and to explore the underlying molecular mechanisms. Apocynin, a Nox inhibitor, was given at 20 mg/kg for 30 min before SAP induction by a retrograde pancreatic duct injection of 5% sodium taurocholate. Histopathological staining, Nox activity and protein expression, oxidative stress markers, apoptosis and associated proteins, cardiac-related enzyme indexes, and cardiac function were assessed in the myocardium in SAP rats. The redox-sensitive MAPK signaling molecules were also examined by western blotting. SAP rats exhibited significant cardiac impairment along with increased Nox activity and protein expression, ROS production, cell apoptosis, and proapoptotic Bax and cleaved caspase-3 protein levels. Notably, Nox inhibition with apocynin prevented SAP-associated cardiac injury evidenced by a decreased histopathologic score, cardiac-related enzymes, and cardiac function through the reduction of ROS production and cell apoptosis. This protective role was further confirmed by a simulation experiment in vitro. Moreover, we found that SAP-induced activation in MAPK signaling molecules in cardiomyocytes was significantly attenuated by Nox inhibition. Our data provide the first evidence that Nox hyperactivation acts as the main source of ROS production in the myocardium, increases oxidative stress, and promotes cell apoptosis via activating the MAPK pathway, which ultimately results in cardiac injury in SAP.
Related collections
Most cited references36
- Record: found
- Abstract: found
- Article: not found
Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system.
- Record: found
- Abstract: found
- Article: not found