- Record: found
- Abstract: found
- Article: found
Stepwise phosphorylation of p65 promotes NF-κB activation and NK cell responses during target cell recognition

Abstract
NF-κB is a key transcription factor that dictates the outcome of diverse immune responses. How NF-κB is regulated by multiple activating receptors that are engaged during natural killer (NK)-target cell contact remains undefined. Here we show that sole engagement of NKG2D, 2B4 or DNAM-1 is insufficient for NF-κB activation. Rather, cooperation between these receptors is required at the level of Vav1 for synergistic NF-κB activation. Vav1-dependent synergistic signalling requires a separate PI3K-Akt signal, primarily mediated by NKG2D or DNAM-1, for optimal p65 phosphorylation and NF-κB activation. Vav1 controls downstream p65 phosphorylation and NF-κB activation. Synergistic signalling is defective in X-linked lymphoproliferative disease (XLP1) NK cells entailing 2B4 dysfunction and required for p65 phosphorylation by PI3K-Akt signal, suggesting stepwise signalling checkpoint for NF-κB activation. Thus, our study provides a framework explaining how signals from different activating receptors are coordinated to determine specificity and magnitude of NF-κB activation and NK cell responses.
Abstract
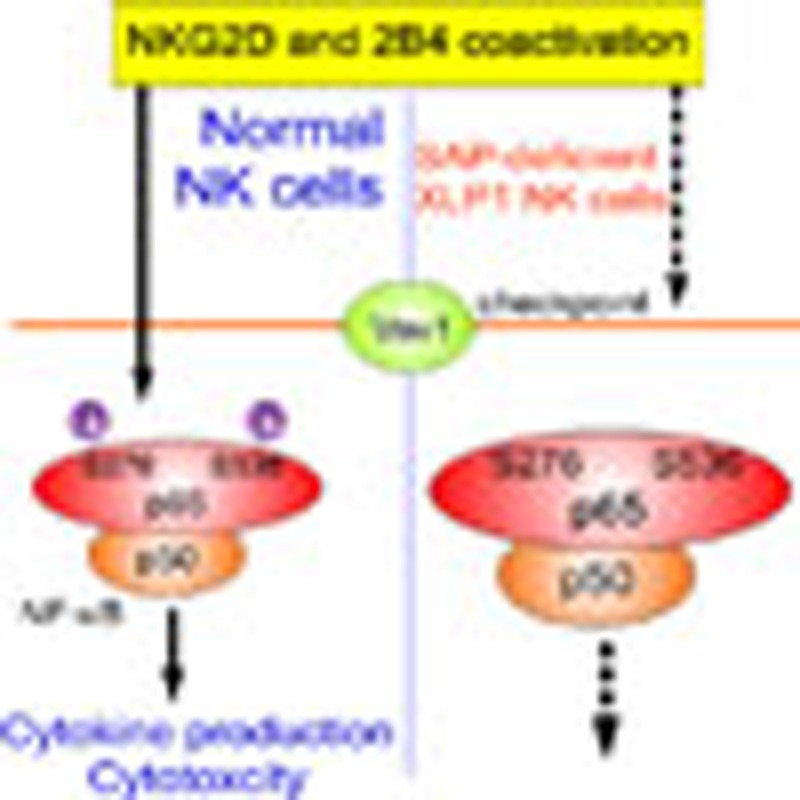 NK cell activation requires multiple signals. Here the authors show that while NKG2D,
2B4, or DNAM-1 receptor activation is insufficient to induce cytokine production,
these signals synergize by Vav-1-mediated NF-κB multiphosphorylation, and this signaling
checkpoint is defective in X-linked lymphoproliferative disease.
NK cell activation requires multiple signals. Here the authors show that while NKG2D,
2B4, or DNAM-1 receptor activation is insufficient to induce cytokine production,
these signals synergize by Vav-1-mediated NF-κB multiphosphorylation, and this signaling
checkpoint is defective in X-linked lymphoproliferative disease.
Related collections
Most cited references52
- Record: found
- Abstract: found
- Article: not found
Functions of natural killer cells.
- Record: found
- Abstract: found
- Article: not found
Up on the tightrope: natural killer cell activation and inhibition.
- Record: found
- Abstract: found
- Article: not found