- Record: found
- Abstract: found
- Article: found
Induction of Monocyte Chemoattractant Proteins in Macrophages via the Production of Granulocyte/Macrophage Colony-Stimulating Factor by Breast Cancer Cells
Read this article at
Abstract
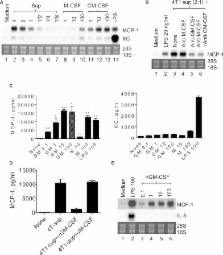
Monocyte chemoattractant protein-1 (MCP-1)/CCL2 plays an important role in the initiation and progression of cancer. We previously reported that in 4T1 murine breast cancer, non-tumor stromal cells, including macrophages, were the major source of MCP-1. In the present study, we analyzed the potential mechanisms by which MCP-1 is upregulated in macrophages infiltrating 4T1 tumors. We found that cell-free culture supernatants of 4T1 cells (4T1-sup) markedly upregulated MCP-1 production by peritoneal inflammatory macrophages. 4T1-sup also upregulated other MCPs, such as MCP-3/CCL7 and MCP-5/CCL12, but modestly upregulated neutrophil chemotactic chemokines, such as KC/CXCL1 or MIP-2/CXCL2. Physicochemical analysis indicated that an approximately 2–3 kDa 4T1 cell product was responsible for the capacity of 4T1-sup to upregulate MCP-1 expression by macrophages. A neutralizing antibody against granulocyte/macrophage colony-stimulating factor (GM-CSF), but not macrophage CSF, almost completely abrogated MCP-1-inducing activity of 4T1-sup, and recombinant GM-CSF potently upregulated MCP-1 production by macrophages. The expression levels of GM-CSF in 4T1 tumors in vivo were higher than other tumors, such as Lewis lung carcinoma. Treatment of mice with anti-GM-CSF antibody significantly reduced the growth of 4T1 tumors at the injection sites but did not reduce MCP-1 production or lung metastasis in tumor-bearing mice. These results indicate that 4T1 cells have the capacity to directly upregulate MCP-1 production by macrophages by releasing GM-CSF; however, other mechanisms are also involved in increased MCP-1 levels in the 4T1 tumor microenvironment.
Related collections
Most cited references39
- Record: found
- Abstract: found
- Article: not found
Conditional gene targeting in macrophages and granulocytes using LysMcre mice.
- Record: found
- Abstract: found
- Article: not found
Colony-stimulating factors in inflammation and autoimmunity.
- Record: found
- Abstract: found
- Article: not found