- Record: found
- Abstract: found
- Article: found
Resistance to the Cyclotide Cycloviolacin O2 in Salmonella enterica Caused by Different Mutations That Often Confer Cross-Resistance or Collateral Sensitivity to Other Antimicrobial Peptides
Read this article at
ABSTRACT
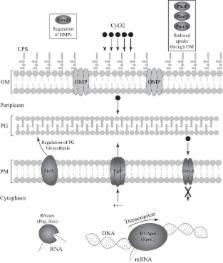
Antimicrobial peptides (AMPs) are essential components of innate immunity in all living organisms, and these potent broad-spectrum antimicrobials have inspired several antibacterial development programs in the past 2 decades. In this study, the development of resistance to the Gram-negative bacterium-specific peptide cycloviolacin O2 (cyO2), a member of the cyclotide family of plant miniproteins, was characterized in Salmonella enterica serovar Typhimurium LT2. Mutants isolated from serial passaging experiments in increasing concentrations of cyO2 were characterized by whole-genome sequencing. The identified mutations were genetically reconstituted in a wild-type background. The additive effect of mutations was studied in double mutants. Fitness costs, levels of resistance, and cross-resistance to another cyclotide, other peptide and nonpeptide antibiotics, and AMPs were determined. A variety of resistance mutations were identified. Some of these reduced fitness and others had no effect on fitness in vitro, in the absence of cyO2. In mouse competition experiments, four of the cyO2-resistant mutants showed a significant fitness advantage, whereas the effects of the mutations in the others appeared to be neutral. The level of resistance was increased by combining several individual resistance mutations. Several cases of cross-resistance and collateral sensitivity between cyclotides, other AMPs, and antibiotics were identified. These results show that resistance to cyclotides can evolve via several different types of mutations with only minor fitness costs and that these mutations often affect resistance to other AMPs.
Related collections
Most cited references48
- Record: found
- Abstract: found
- Article: not found
Mechanisms and consequences of bacterial resistance to antimicrobial peptides.
- Record: found
- Abstract: found
- Article: not found
Use of collateral sensitivity networks to design drug cycling protocols that avoid resistance development.
- Record: found
- Abstract: found
- Article: not found