- Record: found
- Abstract: found
- Article: found
The Role of Retinoic Acid (RA) in Spermatogonial Differentiation1
Read this article at
Abstract
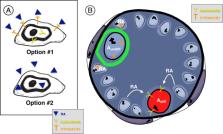
Retinoic acid (RA) directs the sequential, but distinct, programs of spermatogonial differentiation and meiotic differentiation that are both essential for the generation of functional spermatozoa. These processes are functionally and temporally decoupled, as they occur in distinct cell types that arise over a week apart, both in the neonatal and adult testis. However, our understanding is limited in terms of what cellular and molecular changes occur downstream of RA exposure that prepare differentiating spermatogonia for meiotic initiation. In this review, we describe the process of spermatogonial differentiation and summarize the current state of knowledge regarding RA signaling in spermatogonia.
Related collections
Most cited references149
- Record: found
- Abstract: found
- Article: not found
DNA methylation of retrotransposon genes is regulated by Piwi family members MILI and MIWI2 in murine fetal testes.
- Record: found
- Abstract: found
- Article: not found
Essential role of Plzf in maintenance of spermatogonial stem cells.
- Record: found
- Abstract: found
- Article: not found