- Record: found
- Abstract: found
- Article: found
Ca v1.3‐selective inhibitors of voltage‐gated L‐type Ca 2+ channels: Fact or (still) fiction?
Read this article at
Abstract
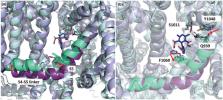
Voltage‐gated L‐type Ca 2+‐channels (LTCCs) are the target of Ca 2+‐channel blockers (CCBs), which are in clinical use for the evidence‐based treatment of hypertension and angina. Their cardiovascular effects are largely mediated by the Ca v1.2‐subtype. However, based on our current understanding of their physiological and pathophysiological roles, Ca v1.3 LTCCs also appear as attractive drug targets for the therapy of various diseases, including treatment‐resistant hypertension, spasticity after spinal cord injury and neuroprotection in Parkinson's disease. Since CCBs inhibit both Ca v1.2 and Ca v1.3, Ca v1.3‐selective inhibitors would be valuable tools to validate the therapeutic potential of Ca v1.3 channel inhibition in preclinical models. Despite a number of publications reporting the discovery of Ca v1.3‐selective blockers, their selectivity remains controversial. We conclude that at present no pharmacological tools exist that are suitable to confirm or refute a role of Ca v1.3 channels in cellular responses. We also suggest essential criteria for a small molecule to be considered Ca v1.3‐selective.
Related collections
Most cited references89
- Record: found
- Abstract: found
- Article: not found
Selective neuronal vulnerability in Parkinson disease
- Record: found
- Abstract: found
- Article: found
The Physiology, Pathology, and Pharmacology of Voltage-Gated Calcium Channels and Their Future Therapeutic Potential
- Record: found
- Abstract: found
- Article: not found