- Record: found
- Abstract: found
- Article: found
Transcriptomic Study on Ovine Immune Responses to Fasciola hepatica Infection
Read this article at
Abstract
Background
Fasciola hepatica is not only responsible for major economic losses in livestock farming, but is also a major food-borne zoonotic agent, with 180 million people being at risk of infection worldwide. This parasite is sophisticated in manipulating the hosts’ immune system to benefit its own survival. A better understanding of the mechanisms underpinning this immunomodulation is crucial for the development of control strategies such as vaccines.
Methodology/principal findings
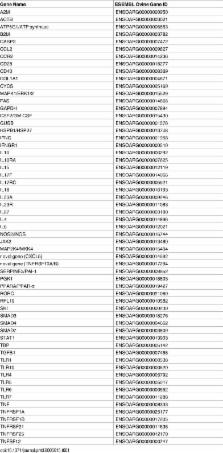
This in vivo study investigated the global gene expression changes of ovine peripheral blood mononuclear cells (PBMC) response to both acute & chronic infection of F. hepatica, and revealed 6490 and 2364 differential expressed genes (DEGS), respectively. Several transcriptional regulators were predicted to be significantly inhibited (e.g. IL12 and IL18) or activated (e.g. miR155-5p) in PBMC during infection. Ingenuity Pathway Analysis highlighted a series of immune-associated pathways involved in the response to infection, including ‘Transforming Growth Factor Beta (TGFβ) signaling’, ‘Production of Nitric Oxide in Macrophages’, ‘Toll-like Receptor (TLRs) Signaling’, ‘Death Receptor Signaling’ and ‘ IL17 Signaling’. We hypothesize that activation of pathways relevant to fibrosis in ovine chronic infection, may differ from those seen in cattle. Potential mechanisms behind immunomodulation in F. hepatica infection are a discussed.
Significance
In conclusion, the present study performed global transcriptomic analysis of ovine PBMC, the primary innate/adaptive immune cells, in response to infection with F. hepatica, using deep-sequencing (RNAseq). This dataset provides novel information pertinent to understanding of the pathological processes in fasciolosis, as well as a base from which to further refine development of vaccines.
Author Summary
Fasciola hepatica (liver fluke) is not only of major health, welfare and economic importance in ruminants, but also an emerging zoonosis. This parasite is sophisticated in manipulating the host’s immune system to benefit its own survival. In this study we investigated global gene expression changes of the primary innate/adaptive immunity-related cells (peripheral blood mononuclear cells) from sheep pre- and post- infected with F. hepatica, which revealed the underpinning mechanisms behind various aspects of fluke-induced immunomodulation, including fibrosis, nitric oxide production, regulation of Toll-like receptors, apoptosis of immune cells, and Th17 differentiation. We hypothesis that activation of pathways relevant to fibrosis in ovine chronic infection, may differ from those seen in cattle. The dataset provided here provides information pertinent to understanding of the immune response to sheep fasciolosis. Due to the lack of studies on human fasciolosis, this information is also valuable for exploring human immune response to F. hepatica infection, as well as for further refine development of vaccines.
Related collections
Most cited references66
- Record: found
- Abstract: found
- Article: found
featureCounts: An efficient general-purpose program for assigning sequence reads to genomic features
- Record: found
- Abstract: found
- Article: not found
Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain.
- Record: found
- Abstract: found
- Article: not found