- Record: found
- Abstract: found
- Article: found
Competitive Redox Chemistries in Vanadium Niobium Oxide for Ultrafast and Durable Lithium Storage

Read this article at
Highlights
-
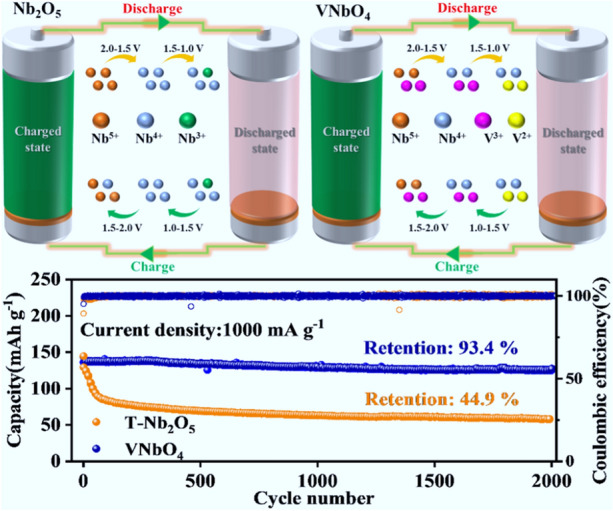
The over-reduction from Nb 5+ to Nb 3+ in the lithiation process have been demonstrated to be the critical reason for the capacity decay of Nb 2O 5 for the first time.
-
A novel competitive redox strategy has been proposed to suppress the over-reduction of Nb 5+ to Nb 3+, which can be achieved by the incorporation of vanadium to form a new rutile VNbO 4 anode.
-
The performance of VNbO 4 anode designed in this study stands among the best in cycle stability.
Abstract
Niobium pentoxide (Nb 2O 5) anodes have gained increasing attentions for high-power lithium-ion batteries owing to the outstanding rate capability and high safety. However, Nb 2O 5 anode suffers poor cycle stability even after modified and the unrevealed mechanisms have restricted the practical applications. Herein, the over-reduction of Nb 5+ has been demonstrated to be the critical reason for the capacity loss for the first time. Besides, an effective competitive redox strategy has been developed to solve the rapid capacity decay of Nb 2O 5, which can be achieved by the incorporation of vanadium to form a new rutile VNbO 4 anode. The highly reversible V 3+/V 2+ redox couple in VNbO 4 can effectively inhibit the over-reduction of Nb 5+. Besides, the electron migration from V 3+ to Nb 5+ can greatly increase the intrinsic electronic conductivity for VNbO 4. As a result, VNbO 4 anode delivers a high capacity of 206.1 mAh g −1 at 0.1 A g −1, as well as remarkable cycle performance with a retention of 93.4% after 2000 cycles at 1.0 A g −1. In addition, the assembled lithium-ion capacitor demonstrates a high energy density of 44 Wh kg −1 at 5.8 kW kg −1. In summary, our work provides a new insight into the design of ultra-fast and durable anodes.
Related collections
Most cited references54
- Record: found
- Abstract: not found
- Article: not found
Commercialization of Lithium Battery Technologies for Electric Vehicles
- Record: found
- Abstract: not found
- Article: not found
A Hybrid Supercapacitor Fabricated with a Carbon Nanotube Cathode and a TiO2–B Nanowire Anode
- Record: found
- Abstract: found
- Article: not found