- Record: found
- Abstract: found
- Article: found
Super-resolution microscopy reveals ultra-low CD19 expression on myeloma cells that triggers elimination by CD19 CAR-T
Read this article at
Abstract
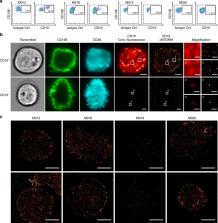
Immunotherapy with chimeric antigen receptor-engineered T-cells (CAR-T) is under investigation in multiple myeloma. There are reports of myeloma remission after CD19 CAR-T therapy, although CD19 is hardly detectable on myeloma cells by flow cytometry (FC). We apply single molecule-sensitive direct stochastic optical reconstruction microscopy ( dSTORM), and demonstrate CD19 expression on a fraction of myeloma cells (10.3–80%) in 10 out of 14 patients (density: 13–5,000 molecules per cell). In contrast, FC detects CD19 in only 2 of these 10 patients, on a smaller fraction of cells. Treatment with CD19 CAR-T in vitro results in elimination of CD19-positive myeloma cells, including those with <100 CD19 molecules per cell. Similar data are obtained by dSTORM analyses of CD20 expression on myeloma cells and CD20 CAR-T. These data establish a sensitivity threshold for CAR-T and illustrate how super-resolution microscopy can guide patient selection in immunotherapy to exploit ultra-low density antigens.
Abstract
CD19 CAR-T cells have achieved some success in treating myeloma patients despite the limited detection of the CD19 antigen. Here, the authors show using dSTORM that 10/14 myeloma samples studied express ultra-low levels of CD19, which are sufficient for engaging CAR-T cells in vitro.
Related collections
Most cited references15
- Record: found
- Abstract: found
- Article: not found
CD22-CAR T Cells Induce Remissions in CD19-CAR Naïve and Resistant B-ALL
- Record: found
- Abstract: found
- Article: not found
Direct stochastic optical reconstruction microscopy with standard fluorescent probes.
- Record: found
- Abstract: found
- Article: not found