- Record: found
- Abstract: found
- Article: found
Lactobacillus rhamnosus Strains Relieve Loperamide-Induced Constipation via Different Pathways Independent of Short-Chain Fatty Acids
Read this article at
Abstract
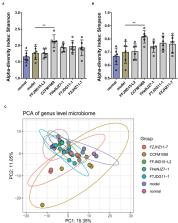
Increasing researches have confirmed the relationship between slow-transit constipation and gut microbiota dysbiosis. Many population and animal experiments have identified probiotics as effectors for the relief of constipation symptoms, but the specific mechanism remains unclear. In this intervention study, Lactobacillus rhamnosus strains isolated from five different sources were administered to mice with loperamide-induced constipation, and the impacts of these strains on constipation-related indicators were evaluated. All five strains of L. rhamnosus were found to improve constipation to various degrees. However, contrary to previous studies, the abilities of L. rhamnosus strains to improve constipation symptoms were not associated with the levels of short-chain fatty acids (SCFAs) in the colon. The effects of different strains of L. rhamnosus on constipation relief were associated with different aspects of the GI tract, including gastrointestinal regulatory peptides, neurotransmitters, neurotrophic factors, and gut microbiota. The findings of this study demonstrate that L. rhamnosus strains can alleviate constipation-related symptoms via different pathways independent of SCFAs regulation. This study yields a new perspective for clinical use of probiotics to better improve constipation symptoms, by combining strains with different mechanisms for alleviation of constipation.
Related collections
Most cited references29
- Record: found
- Abstract: found
- Article: not found
Meta-analyses of human gut microbes associated with obesity and IBD.
- Record: found
- Abstract: found
- Article: not found
Gut Microbiota-Produced Tryptamine Activates an Epithelial G-Protein-Coupled Receptor to Increase Colonic Secretion
- Record: found
- Abstract: found
- Article: not found