- Record: found
- Abstract: found
- Article: found
Myocardial fibrosis: biomedical research from bench to bedside
Read this article at
Abstract
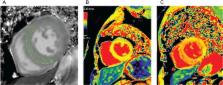
Myocardial fibrosis refers to a variety of quantitative and qualitative changes in the interstitial myocardial collagen network that occur in response to cardiac ischaemic insults, systemic diseases, drugs, or any other harmful stimulus affecting the circulatory system or the heart itself. Myocardial fibrosis alters the architecture of the myocardium, facilitating the development of cardiac dysfunction, also inducing arrhythmias, influencing the clinical course and outcome of heart failure patients. Focusing on myocardial fibrosis may potentially improve patient care through the targeted diagnosis and treatment of emerging fibrotic pathways. The European Commission funded the FIBROTARGETS consortium as a multinational academic and industrial consortium with the primary aim of performing a systematic and collaborative search of targets of myocardial fibrosis, and then translating these mechanisms into individualized diagnostic tools and specific therapeutic pharmacological options for heart failure. This review focuses on those methodological and technological aspects considered and developed by the consortium to facilitate the transfer of the new mechanistic knowledge on myocardial fibrosis into potential biomedical applications.
Related collections
Most cited references59
- Record: found
- Abstract: found
- Article: not found
Swine as models in biomedical research and toxicology testing.
- Record: found
- Abstract: found
- Article: not found
Myofibroblast-mediated mechanisms of pathological remodelling of the heart.

- Record: found
- Abstract: found
- Article: found