- Record: found
- Abstract: found
- Article: found
Oxygenation of adipose tissue: A human perspective
Read this article at
Abstract
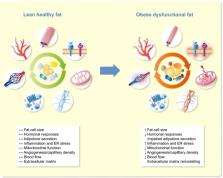
Obesity is a complex disorder of excessive adiposity, and is associated with adverse health effects such as cardiometabolic complications, which are to a large extent attributable to dysfunctional white adipose tissue. Adipose tissue dysfunction is characterized by adipocyte hypertrophy, impaired adipokine secretion, a chronic low‐grade inflammatory status, hormonal resistance and altered metabolic responses, together contributing to insulin resistance and related chronic diseases. Adipose tissue hypoxia, defined as a relative oxygen deficit, in obesity has been proposed as a potential contributor to adipose tissue dysfunction, but studies in humans have yielded conflicting results. Here, we will review the role of adipose tissue oxygenation in the pathophysiology of obesity‐related complications, with a specific focus on human studies. We will provide an overview of the determinants of adipose tissue oxygenation, as well as the role of adipose tissue oxygenation in glucose homeostasis, lipid metabolism and inflammation. Finally, we will discuss the putative effects of physiological and experimental hypoxia on adipose tissue biology and whole‐body metabolism in humans. We conclude that several lines of evidence suggest that alteration of adipose tissue oxygenation may impact metabolic homeostasis, thereby providing a novel strategy to combat chronic metabolic diseases in obese humans.
Related collections
Most cited references143
- Record: found
- Abstract: found
- Article: not found
Origin and physiological roles of inflammation.
- Record: found
- Abstract: found
- Article: not found
Cloning of adiponectin receptors that mediate antidiabetic metabolic effects.
- Record: found
- Abstract: found
- Article: not found