- Record: found
- Abstract: found
- Article: found
p65BTK is a novel potential actionable target in KRAS-mutated/EGFR-wild type lung adenocarcinoma
Read this article at
Abstract
Background
Lung cancer is still the main cause of cancer death worldwide despite the availability of targeted therapies and immune-checkpoint inhibitors combined with chemotherapy. Cancer cell heterogeneity and primary or acquired resistance mechanisms cause the elusive behaviour of this cancer and new biomarkers and active drugs are urgently needed to overcome these limitations. p65BTK, a novel isoform of the Bruton Tyrosine Kinase may represent a new actionable target in non-small cell lung cancer (NSCLC).
Methods
p65BTK expression was evaluated by immunohistochemistry in 382 NSCLC patients with complete clinico-pathological records including smoking habit, ALK and EGFR status, and in metastatic lymph nodes of 30 NSCLC patients. NSCLC cell lines mutated for p53 and/or a component of the RAS/MAPK pathway and primary lung cancer-derived cells from Kras/Trp53 null mice were used as a preclinical model. The effects of p65BTK inhibition by BTK Tyrosine Kinase Inhibitors (TKIs) (Ibrutinib, AVL-292, RN486) and first-generation EGFR-TKIs (Gefitinib, Erlotinib) on cell viability were evaluated by MTT. The effects of BTK-TKIs on cell growth and clonogenicity were assessed by crystal violet and colony assays, respectively. Cell toxicity assays were performed to study the effect of the combination of non-toxic concentrations of BTK-TKIs with EGFR-TKIs and standard-of-care (SOC) chemotherapy (Cisplatin, Gemcitabine, Pemetrexed).
Results
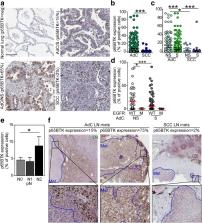
p65BTK was significantly over-expressed in EGFR-wild type (wt) adenocarcinomas (AdC) from non-smoker patients and its expression was also preserved at the metastatic site. p65BTK was also over-expressed in cell lines mutated for KRAS or for a component of the RAS/MAPK pathway and in tumors from Kras/Trp53 null mice. BTK-TKIs were more effective than EGFR-TKIs in decreasing cancer cell viability and significantly impaired cell proliferation and clonogenicity. Moreover, non-toxic doses of BTK-TKIs re-sensitized drug-resistant NSCLC cell lines to both target- and SOC therapy, independently from EGFR/KRAS status.
Conclusions
p65BTK results as an emerging actionable target in non-smoking EGFR-wt AdC, also at advanced stages of disease. Notably, these patients are not eligible for EGFR-TKIs-based therapy due to a lack of EGFR mutation. The combination of BTK-TKIs with EGFR-TKIs is cytotoxic for EGFR-wt/KRAS-mutant/p53-null tumors and BTK-TKIs re-sensitizes drug-resistant NSCLC to SOC chemotherapy. Therefore, our data suggest that adding BTK-TKIs to SOC chemotherapy and EGFR-targeted therapy may open new avenues for clinical trials in currently untreatable NSCLC.
Related collections
Most cited references36
- Record: found
- Abstract: found
- Article: not found
Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib.

- Record: found
- Abstract: found
- Article: found