- Record: found
- Abstract: found
- Article: found
S100 Proteins As an Important Regulator of Macrophage Inflammation
Read this article at
Abstract
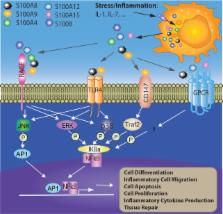
The S100 proteins, a family of calcium-binding cytosolic proteins, have a broad range of intracellular and extracellular functions through regulating calcium balance, cell apoptosis, migration, proliferation, differentiation, energy metabolism, and inflammation. The intracellular functions of S100 proteins involve interaction with intracellular receptors, membrane protein recruitment/transportation, transcriptional regulation and integrating with enzymes or nucleic acids, and DNA repair. The S100 proteins could also be released from the cytoplasm, induced by tissue/cell damage and cellular stress. The extracellular S100 proteins, serving as a danger signal, are crucial in regulating immune homeostasis, post-traumatic injury, and inflammation. Extracellular S100 proteins are also considered biomarkers for some specific diseases. In this review, we will discuss the multi-functional roles of S100 proteins, especially their potential roles associated with cell migration, differentiation, tissue repair, and inflammation.
Related collections
Most cited references147
- Record: found
- Abstract: found
- Article: not found
Altered macrophage differentiation and immune dysfunction in tumor development.
- Record: found
- Abstract: found
- Article: not found
RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides.

- Record: found
- Abstract: found
- Article: found
