- Record: found
- Abstract: found
- Article: found
Role of TRPM2 in H 2O 2-Induced Cell Apoptosis in Endothelial Cells
Read this article at
Abstract
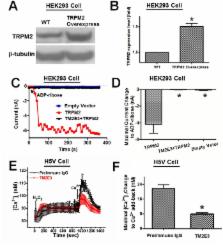
Melastatin-like transient receptor potential channel 2 (TRPM2) is an oxidant-sensitive and cationic non-selective channel that is expressed in mammalian vascular endothelium. Here we investigated the functional role of TRPM2 channels in hydrogen peroxide (H 2O 2)-induced cytosolic Ca 2+ ([Ca 2+] i) elavation, whole-cell current increase, and apoptotic cell death in murine heart microvessel endothelial cell line H5V. A TRPM2 blocking antibody (TM2E3), which targets the E3 region near the ion permeation pore of TRPM2, was developed. Treatment of H5V cells with TM2E3 reduced the [Ca 2+] i rise and whole-cell current change in response to H 2O 2. Suppressing TRPM2 expression using TRPM2-specific short hairpin RNA (shRNA) had similar inhibitory effect. H 2O 2-induced apoptotic cell death in H5V cells was examined using MTT assay, DNA ladder formation analysis, and DAPI-based nuclear DNA condensation assay. Based on these assays, TM2E3 and TRPM2-specific shRNA both showed protective effect against H 2O 2-induced apoptotic cell death. TM2E3 and TRPM2-specific shRNA also protect the cells from tumor necrosis factor (TNF)-α-induced cell death in MTT assay. In contrast, overexpression of TRPM2 in H5V cells resulted in an increased response in [Ca 2+] i and whole-cell currents to H 2O 2. TRPM2 overexpression also aggravated the H 2O 2-induced apoptotic cell death. Downstream pathways following TRPM2 activation was examined. Results showed that TRPM2 activity stimulated caspase-8, caspase-9 and caspase-3. These findings strongly suggest that TRPM2 channel mediates cellular Ca 2+ overload in response to H 2O 2 and contribute to oxidant-induced apoptotic cell death in vascular endothelial cells. Down-regulating endogenous TRPM2 could be a means to protect the vascular endothelial cells from apoptotic cell death.
Related collections
Most cited references36
- Record: found
- Abstract: found
- Article: not found
ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology.
- Record: found
- Abstract: found
- Article: not found
Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture).
- Record: found
- Abstract: found
- Article: not found