- Record: found
- Abstract: found
- Article: found
Liver structural transformation after partial hepatectomy and repeated partial hepatectomy in rats: A renewed view on liver regeneration
Read this article at
Abstract
BACKGROUND
The phenomenon of liver regeneration after partial hepatectomy (PH) is still a subject of considerable interest due to the increasing frequency of half liver transplantation on the one hand, and on the other hand, new surgical approaches which allow removal of massive space-occupying hepatic tumors, which earlier was considered as inoperable. Interestingly, the mechanisms of liver regeneration are extensively studied after PH but less attention is paid to the architectonics of the regenerated organ. Because of this, the question “How does the structure of regenerated liver differ from normal, regular liver?” has not been fully answered yet. Furthermore, almost without any attention is left the liver's structural transformation after repeated hepatectomy (of the re-regenereted liver).
AIM
To compare the architectonics of the lobules and circulatory bed of normal, re-generated and re-regenerated livers.
METHODS
The livers of 40 adult, male, albino Wistar rats were studied. 14 rats were subjected to PH - the 1 st study group (SG1); 10 rats underwent repeated PH – the 2 nd study group (SG2); 16 rats were subjected to sham operation - control group (CG); The livers were studied after 9 months from PH, and after 6 months from repeated PH. Cytological (Schiff reaction for the determination of DNA concen-tration), histological (H&E, Masson trichrome, CK8 Immunohistochemical marker, transparent slides after Indian Ink injection, ), morphometrical (hepatocytes areas, perimeters and ploidy) and Electron Microscopical (Scanning Electron Microscopy of corrosion casts) methods were used.
RESULTS
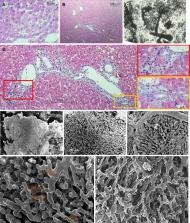
In the SG1 and SG2, the area of hepatocytes and their perimeter are increased compared to the CG ( P < 0.05). However, the areas and perimeters of the hepatocytes of the SG 1 and SG 2 groups reveal a lesser difference. In regenerated (SG 1) and re-regenerated (SG 2) livers, the hepatocytes form the remodeled lobules, which size (300-1200 µm) exceeds the sizes of the lobules from CG (300-600 µm). The remodeled lobules (especially the “mega-lobules” with the sizes 1000-1200 µm) contain the transformed meshworks of the sinusoids, the part of which is dilated asymmetrically. This meshwork might have originated from the several portal venules (interlobular and/or inlet). The boundaries between the adjacent lobules (including mega-lobules) are widened and filled by connective tissue fibers, which gives the liver parenchyma a nodular look. In SG 2 the unevenness of sinusoid diameters, as well as the boundaries between the lobules (including the mega-lobules) are more vividly expressed in comparison with SG 1. The liver tissue of both SG 1 and SG 2 is featured by the slightly expressed ductular reaction.
Related collections
Most cited references53
- Record: found
- Abstract: found
- Article: not found
Liver regeneration.
- Record: found
- Abstract: found
- Article: not found