- Record: found
- Abstract: found
- Article: found
FOXP3 and Tip60 Structural Interactions Relevant to IPEX Development Lead to Potential Therapeutics to Increase FOXP3 Dependent Suppressor T Cell Functions
Read this article at
Abstract
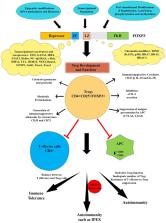
Regulatory T (Treg) cells play a role in the maintenance of immune homeostasis and are critical mediators of immune tolerance. The Forkhead box P3 (FOXP3) protein acts as a regulator for Treg development and function. Mutations in the FOXP3 gene can lead to autoimmune diseases such as Immunodysregulation, polyendocrinopathy, enteropathy, and X-linked (IPEX) syndrome in humans, often resulting in death within the first 2 years of life and a scurfy like phenotype in Foxp3 mutant mice. We discuss biochemical features of the FOXP3 ensemble including its regulation at various levels (epigenetic, transcriptional, and post-translational modifications) and molecular functions. The studies also highlight the interactions of FOXP3 and Tat-interacting protein 60 (Tip60), a principal histone acetylase enzyme that acetylates FOXP3 and functions as an essential subunit of the FOXP3 repression ensemble complex. Lastly, we have emphasized the role of allosteric modifiers that help stabilize FOXP3:Tip60 interactions and discuss targeting this interaction for the therapeutic manipulation of Treg activity.
Related collections
Most cited references109
- Record: found
- Abstract: found
- Article: not found
Foxp3 programs the development and function of CD4+CD25+ regulatory T cells.
- Record: found
- Abstract: found
- Article: not found
Control of regulatory T cell development by the transcription factor Foxp3.
- Record: found
- Abstract: found
- Article: not found
