- Record: found
- Abstract: found
- Article: found
The Nucleolus: In Genome Maintenance and Repair
Read this article at
Abstract
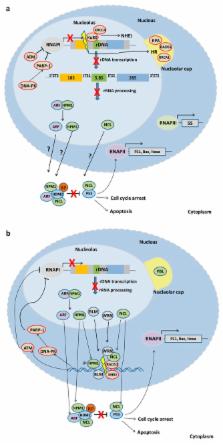
The nucleolus is the subnuclear membrane-less organelle where rRNA is transcribed and processed and ribosomal assembly occurs. During the last 20 years, however, the nucleolus has emerged as a multifunctional organelle, regulating processes that go well beyond its traditional role. Moreover, the unique organization of rDNA in tandem arrays and its unusually high transcription rates make it prone to unscheduled DNA recombination events and frequent RNA:DNA hybrids leading to DNA double strand breaks (DSBs). If not properly repaired, rDNA damage may contribute to premature disease onset and aging. Deregulation of ribosomal synthesis at any level from transcription and processing to ribosomal subunit assembly elicits a stress response and is also associated with disease onset. Here, we discuss how genome integrity is maintained within nucleoli and how such structures are functionally linked to nuclear DNA damage response and repair giving an emphasis on the newly emerging roles of the nucleolus in mammalian physiology and disease.
Related collections
Most cited references140
- Record: found
- Abstract: found
- Article: not found
Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis.
- Record: found
- Abstract: found
- Article: not found
p53 has a direct apoptogenic role at the mitochondria.
- Record: found
- Abstract: found
- Article: not found