- Record: found
- Abstract: found
- Article: found
Myeloid‐derived suppressor cells support remyelination in a murine model of multiple sclerosis by promoting oligodendrocyte precursor cell survival, proliferation, and differentiation
Read this article at
Abstract
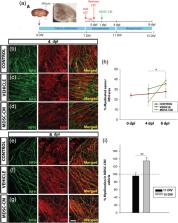
The most frequent variant of multiple sclerosis (MS) is the relapsing–remitting form, characterized by symptomatic phases followed by periods of total/partial recovery. Hence, it is possible that these patients can benefit from endogenous agents that control the inflammatory process and favor spontaneous remyelination. In this context, there is increasing interest in the role of myeloid‐derived suppressor cells (MDSCs) during the clinical course of experimental autoimmune encephalomyelitis (EAE). MDSCs speed up infiltrated T‐cell anergy and apoptosis. In different animal models of MS, a milder disease course is related to higher presence/density of MDSCs in the periphery, and smaller demyelinated lesions in the central nervous system (CNS). These observations lead us to wonder whether MDSCs might not only exert an anti‐inflammatory effect but might also have direct influence on oligodendrocyte precursor cells (OPCs) and remyelination. In the present work, we reveal for the first time the relationship between OPCs and MDSCs in EAE, relationship that is guided by the distance from the inflammatory core. We describe the effects of MDSCs on survival, proliferation, as well as potent promoters of OPC differentiation toward mature phenotypes. We show for the first time that osteopontin is remarkably present in the analyzed secretome of MDSCs. The ablation of this cue from MDSCs‐secretome demonstrates that osteopontin is the main MDSC effector on these oligodendroglial cells. These data highlight a crucial pathogenic interaction between innate immunity and the CNS, opening ways to develop MDSC‐ and/or osteopontin‐based therapies to promote effective myelin preservation and repair in MS patients.
Main Points
-
The abundance of myeloid‐derived suppressor cells (MDSCs) correlates with the density of oligodendrocyte precursor cells (OPCs) in experimental autoimmune encephalomyelitis demyelinating lesions.
-
MDSC secretome favors OPC survival, proliferation, and differentiation toward myelinating phenotypes.
-
Osteopontin is crucial in MDSCs secretome to act on OPC biology.
Related collections
Most cited references82

- Record: found
- Abstract: found
- Article: found
Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards
- Record: found
- Abstract: found
- Article: not found
Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage.
- Record: found
- Abstract: found
- Article: not found
