- Record: found
- Abstract: found
- Article: found
The prospect of serum and glucocorticoid-inducible kinase 1 (SGK1) in cancer therapy: a rising star
Read this article at
Abstract
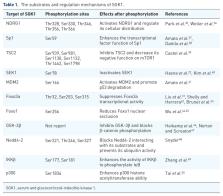
Serum and glucocorticoid-inducible kinase 1 (SGK1) is an AGC kinase that has been reported to be involved in a variety of physiological and pathological processes. Recent evidence has accumulated that SGK1 acts as an essential Akt-independent mediator of phosphatidylinositol 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) signaling pathway in cancer. SGK1 is overexpressed in several tumors, including prostate cancer, colorectal carcinoma, glioblastoma, breast cancer, and endometrial cancer. The functions of SGK1 include regulating tumor growth, survival, metastasis, autophagy, immunoregulation, calcium (Ca 2+) signaling, cancer stem cells, cell cycle, and therapeutic resistance. In this review, we introduce the pleiotropic role of SGK1 in the development and progression of tumors, summarize its downstream targets, and integrate the knowledge provided by preclinical studies that the prospect of SGK1 inhibition as a potential therapeutic approach.
Related collections
Most cited references97
- Record: found
- Abstract: found
- Article: not found
Regulatory T cells in cancer immunotherapy
- Record: found
- Abstract: found
- Article: not found
Store-operated calcium channels.
- Record: found
- Abstract: found
- Article: not found