- Record: found
- Abstract: found
- Article: found
Mapping the Evolution of Hypervirulent Klebsiella pneumoniae
Read this article at
ABSTRACT
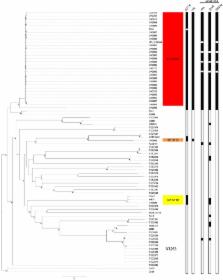
Highly invasive, community-acquired Klebsiella pneumoniae infections have recently emerged, resulting in pyogenic liver abscesses. These infections are caused by hypervirulent K. pneumoniae (hvKP) isolates primarily of capsule serotype K1 or K2. Hypervirulent K1 isolates belong to clonal complex 23 (CC23), indicating that this clonal lineage has a specific genetic background conferring hypervirulence. Here, we apply whole-genome sequencing to a collection of K. pneumoniae isolates to characterize the phylogenetic background of hvKP isolates with an emphasis on CC23. Most of the hvKP isolates belonged to CC23 and grouped into a distinct monophyletic clade, revealing that CC23 is a unique clonal lineage, clearly distinct from nonhypervirulent strains. Separate phylogenetic analyses of the CC23 isolates indicated that the CC23 lineage evolved recently by clonal expansion from a single common ancestor. Limited grouping according to geographical origin was observed, suggesting that CC23 has spread globally through multiple international transmissions. Conversely, hypervirulent K2 strains clustered in genetically unrelated groups. Strikingly, homologues of a large virulence plasmid were detected in all hvKP clonal lineages, indicating a key role in K. pneumoniae hypervirulence. The plasmid encodes two siderophores, aerobactin and salmochelin, and RmpA (regulator of the mucoid phenotype); all these factors were found to be restricted to hvKP isolates. Genomic comparisons revealed additional factors specifically associated with CC23. These included a distinct variant of a genomic island encoding yersiniabactin, colibactin, and microcin E492. Furthermore, additional novel genomic regions unique to CC23 were revealed which may also be involved in the increased virulence of this important clonal lineage.
IMPORTANCE
During the last 3 decades, hypervirulent Klebsiella pneumoniae (hvKP) isolates have emerged, causing severe community-acquired infections primarily in the form of pyogenic liver abscesses. This syndrome has so far primarily been found in Southeast Asia, but increasing numbers of cases are being reported worldwide, indicating that the syndrome is turning into a globally emerging disease. We applied whole-genome sequencing to a collection of K. pneumoniae clinical isolates to reveal the phylogenetic background of hvKP and to identify genetic factors associated with the increased virulence. The hvKP isolates primarily belonged to clonal complex 23 (CC23), and this clonal lineage was revealed to be clearly distinct from nonhypervirulent strains. A specific virulence plasmid was found to be associated with hypervirulence, and novel genetic determinants uniquely associated with CC23 were identified. Our findings extend the understanding of the genetic background of the emergence of hvKP clones.
Related collections
Most cited references64

- Record: found
- Abstract: found
- Article: found
Hypervirulent (hypermucoviscous) Klebsiella pneumoniae
- Record: found
- Abstract: found
- Article: not found
Klebsiella pneumoniae liver abscess: a new invasive syndrome.
- Record: found
- Abstract: found
- Article: not found
A Novel Virulence Gene in Klebsiella pneumoniae Strains Causing Primary Liver Abscess and Septic Metastatic Complications
Author and article information
Comments
Comment on this article
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.