- Record: found
- Abstract: found
- Article: found
HDL-Mediated Cholesterol Efflux and Plasma Loading Capacities Are Altered in Subjects with Metabolically- but Not Genetically Driven Non-Alcoholic Fatty Liver Disease (NAFLD)
Read this article at
Abstract
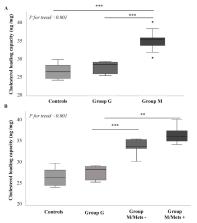
Background. Non-alcoholic fatty liver disease (NAFLD) increases the risk of atherosclerosis but this risk may differ between metabolically- vs. genetically-driven NAFLD. High-density lipoprotein (HDL)-mediated cholesterol efflux (CEC) and plasma loading capacity (CLC) are key factors in atherogenesis. Aims. To test whether CEC and CLC differ between metabolically- vs. genetically-determined NAFLD. Methods: CEC and CLC were measured in 19 patients with metabolic NAFLD and wild-type PNPLA3 genotype (Group M), 10 patients with genetic NAFLD carrying M148M PNPLA3 genotype (Group G), and 10 controls PNPLA3 wild-types and without NAFLD. CEC and CLC were measured ex vivo by isotopic and fluorimetric techniques using cellular models. Results: Compared with Group G, Group M showed reduced total CEC (−18.6%; p < 0.001) as well as that mediated by cholesterol transporters (−25.3% ABCA1; −16.3% ABCG1; −14.8% aqueous diffusion; all p < 0.04). No difference in CEC was found between Group G and controls. The presence of metabolic syndrome further impaired ABCG1-mediated CEC in Group M. Group M had higher plasma-induced CLC than Group G and controls ( p < 0.001). Conclusions: Metabolically-, but not genetically-, driven NAFLD associates with dysfunctional HDL-meditated CEC and abnormal CLC. These data suggest that the mechanisms of anti-atherogenic protection in metabolic NAFLD are impaired.
Related collections
Most cited references45
- Record: found
- Abstract: found
- Article: not found
Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes.
- Record: found
- Abstract: not found
- Article: not found
Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement.
- Record: found
- Abstract: found
- Article: not found