- Record: found
- Abstract: found
- Article: found
Recent Advances on Diels‐Alder‐Driven Preparation of Bio‐Based Aromatics

Read this article at
Abstract
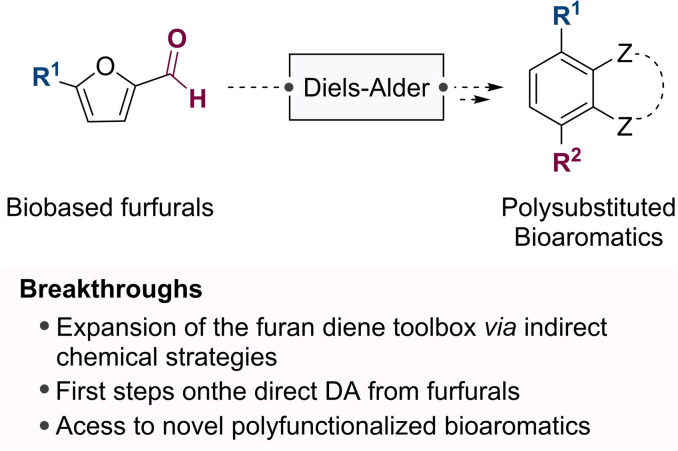
The preparation of high value‐added chemicals from renewable resources is a crucial approach towards a sustainable economy. One prominent alternative to the production of petroleum‐based chemicals from fossil resources is through the sequential Diels‐Alder/aromatization reactions of biomass‐derived furan platforms. This Concept is focused on the recent boom in bio‐based furan DA strategies for aromatization of bio‐based platform chemicals, particularly that of furfurals, ranging from indirect use and activation strategies to recent examples of direct DA reaction of these electron‐withdrawing biomass‐derived furans.
Abstract
Ta‐DA! The Diels‐Alder (DA) reaction of electron‐rich furans with electron‐poor alkenes has been a benchmark for the preparation of complex scaffolds such as 7‐oxanorbornenes. Recently this technology has been used to prepare aromatics from bio‐based furanic platforms. The recently described direct DA reaction of bio‐based furfurals expands the scope of bioaromatic preparation to afford key biomass derivatives.