- Record: found
- Abstract: found
- Article: found
The effects of a genome-wide supported variant in the CACNA1C gene on cortical morphology in schizophrenia patients and healthy subjects
Read this article at
Abstract
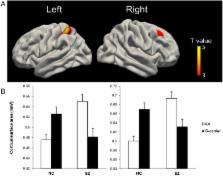
Schizophrenia is a highly heritable disorder with multiple susceptibility genes. Previously, we identified CACNA1C rs2007044 as a new risk locus for schizophrenia, with the minor allele G as risk allele. This association was recently validated by a powerful genome-wide association study. However, the underlying neural mechanisms remain unclear. Therefore, we tested whether the risk allele has an influence on cortical surface area and thickness in a sample of schizophrenia patients and healthy controls. We found significant genotype by diagnosis interactions on cortical surface area, but not thickness, in the right dorsolateral prefrontal cortex and the left superior parietal cortex, both of which are key components of the central executive network. Moreover, the surface areas of both regions were inversely correlated with PANSS negative scores in AA homogeneous patients but not in G-carriers. This is the first study to describe the influence of the new genome-wide supported schizophrenia risk variant on cortical morphology. Our data revealed a significant genetic effect of cortical surface area in pivotal brain regions, which have been implicated in the pathophysiology of schizophrenia, possibly via their involvement in cognitive functions. These results yield new insights into the potential neural mechanisms linking CACNA1C to the risk of schizophrenia.
Related collections
Most cited references43
- Record: found
- Abstract: found
- Article: not found
A TALE nuclease architecture for efficient genome editing.
- Record: found
- Abstract: found
- Article: not found
Specification of cerebral cortical areas.
- Record: found
- Abstract: found
- Article: not found