- Record: found
- Abstract: found
- Article: found
Investigating the Synergistic Potential of Low-Dose HDAC3 Inhibition and Radiotherapy in Alzheimer’s Disease Models
Read this article at
Abstract
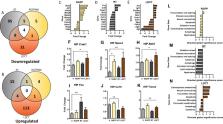
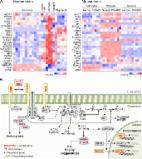
We have previously shown that histone deacetylase (HDAC) inhibition and cranial radiotherapy (RT) independently improve molecular and behavioral Alzheimer’s disease (AD)-like phenotypes. In the present study, we investigate the synergistic potential of using both RT and HDACi as a low-dose combination therapy (LDCT) to maximize disease modification (reduce neuroinflammation and amyloidogenic APP processing, increase neurotrophic gene expression) while minimizing the potential for treatment-associated side effects.
LDCT consisted of daily administration of the HDAC3 inhibitor RGFP966 and/or bi-weekly cranial x-irradiation. Amyloid-beta precursor protein (APP) processing and innate immune response to LDCT were assessed in vitro and in vivo using human and murine cell models and 3xTg-AD mice. After 2 months of LDCT in mice, behavioral analyses as well as expression and modification of key AD-related targets (Aβ, tau, Csf1r, Bdnf, etc.) were assessed in the hippocampus (HIP) and prefrontal cortex (PFC).
LDCT induced a tolerant, anti-inflammatory innate immune response in microglia and increased non-amyloidogenic APP processing in vitro. Both RT and LDCT improved the rate of learning and spatial memory in the Barnes maze test. LDCT induced a unique anti-AD HIP gene expression profile that included upregulation of neurotrophic genes and downregulation of inflammation-related genes. RT lowered HIP Aβ 42/40 ratio and Bace1 protein, while LDCT lowered PFC p-tau181 and HIP Bace1 levels.
Our study supports the rationale for combining complementary therapeutic approaches at low doses to target multifactorial AD pathology synergistically. Namely, LDCT with RGFP966 and cranial RT shows disease-modifying potential against a wide range of AD-related hallmarks.
Related collections
Most cited references70
- Record: found
- Abstract: found
- Article: not found
Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method.
- Record: found
- Abstract: found
- Article: not found
The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases
- Record: found
- Abstract: found
- Article: found