- Record: found
- Abstract: found
- Article: not found
Temperature-induced opening of TRPV1 ion channel is stabilized by the pore domain
Summary
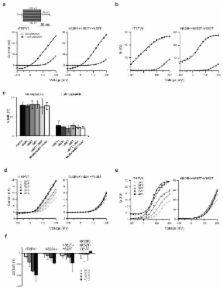
TRPV1 is the founding and best-studied member of the family of temperature-activated transient receptor potential ion channels (thermoTRPs). Voltage, chemicals, and heat amongst other agonists allosterically gate TRPV1. Molecular determinants for TRPV1 activation by capsaicin, allicin, acid, ammonia, and voltage have been identified. However, the structures and mechanisms mediating its pronounced temperature-sensitivity remain unclear. Recent studies of the related channel TRPV3 identified residues within the pore region required for heat activation. Here we use both random and targeted mutagenesis screens of TRPV1 and identify point mutations in the outer pore region that specifically impair temperature-activation. Single channel analysis shows that TRPV1 mutations disrupt heat-sensitivity by ablating long channel openings, that are part of the temperature-gating pathway. We propose that sequential occupancy of short and long open states upon activation provides a mechanism to enhance temperature-sensitivity. Our study suggests that the outer pore plays a general role in heat-sensitivity of thermoTRPs.
Related collections
Most cited references39
- Record: found
- Abstract: found
- Article: not found
Impaired nociception and pain sensation in mice lacking the capsaicin receptor.
- Record: found
- Abstract: found
- Article: not found
A TRP channel that senses cold stimuli and menthol.
- Record: found
- Abstract: found
- Article: not found
