- Record: found
- Abstract: found
- Article: found
Cardiovascular Disease in Duchenne Muscular Dystrophy : Overview and Insight Into Novel Therapeutic Targets

Read this article at
Highlights
-
•
Cardiomyopathy is the leading cause of death in patients with DMD.
-
•
DMD has no cure, and there is no current consensus for treatment of DMD cardiomyopathy.
-
•
This review discusses therapeutic strategies to potentially reduce or prevent cardiac dysfunction in DMD patients.
-
•
Additional studies are needed to firmly establish optimal treatment modalities for DMD cardiomyopathy.
Summary
Duchenne muscular dystrophy (DMD) is a devastating disease affecting approximately 1 in every 3,500 male births worldwide. Multiple mutations in the dystrophin gene have been implicated as underlying causes of DMD. However, there remains no cure for patients with DMD, and cardiomyopathy has become the most common cause of death in the affected population. Extensive research is under way investigating molecular mechanisms that highlight potential therapeutic targets for the development of pharmacotherapy for DMD cardiomyopathy. In this paper, the authors perform a literature review reporting on recent ongoing efforts to identify novel therapeutic strategies to reduce, prevent, or reverse progression of cardiac dysfunction in DMD.
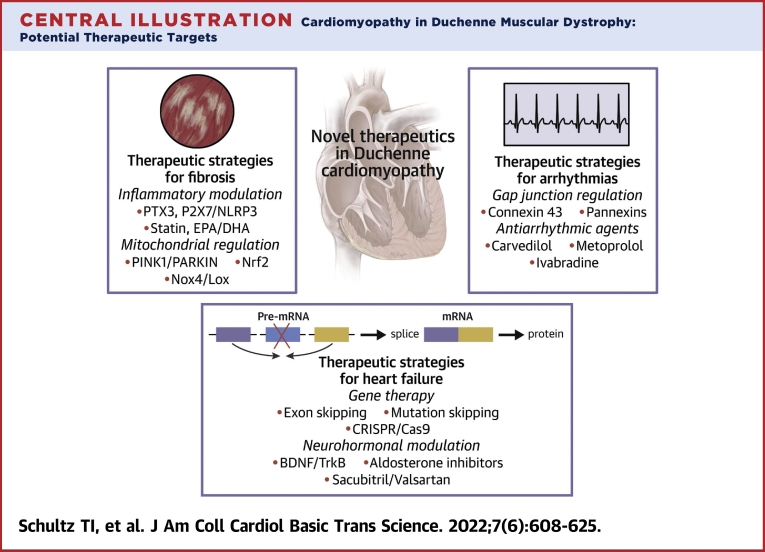
Central Illustration
Related collections
Most cited references143
- Record: found
- Abstract: found
- Article: not found
Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure
- Record: found
- Abstract: found
- Article: not found