- Record: found
- Abstract: found
- Article: found
Dysfunctional LAT2 Amino Acid Transporter Is Associated With Cataract in Mouse and Humans
Read this article at
Abstract
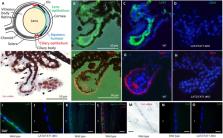
Cataract, the loss of ocular lens transparency, accounts for ∼50% of worldwide blindness and has been associated with water and solute transport dysfunction across lens cellular barriers. We show that neutral amino acid antiporter LAT2 (Slc7a8) and uniporter TAT1 ( Slc16a10) are expressed on mouse ciliary epithelium and LAT2 also in lens epithelium. Correspondingly, deletion of LAT2 induced a dramatic decrease in lens essential amino acid levels that was modulated by TAT1 defect. Interestingly, the absence of LAT2 led to increased incidence of cataract in mice, in particular in older females, and a synergistic effect was observed with simultaneous lack of TAT1. Screening SLC7A8 in patients diagnosed with congenital or age-related cataract yielded one homozygous single nucleotide deletion segregating in a family with congenital cataract. Expressed in HeLa cells, this LAT2 mutation did not support amino acid uptake. Heterozygous LAT2 variants were also found in patients with cataract some of which showed a reduced transport function when expressed in HeLa cells. Whether heterozygous LAT2 variants may contribute to the pathology of cataract needs to be further investigated. Overall, our results suggest that defects of amino acid transporter LAT2 are implicated in cataract formation, a situation that may be aggravated by TAT1 defects.
Related collections
Most cited references46
- Record: found
- Abstract: found
- Article: found
Cat-Map: putting cataract on the map
- Record: found
- Abstract: found
- Article: not found
Identification of a membrane protein, LAT-2, that Co-expresses with 4F2 heavy chain, an L-type amino acid transport activity with broad specificity for small and large zwitterionic amino acids.
- Record: found
- Abstract: found
- Article: not found