- Record: found
- Abstract: found
- Article: found
Potential Involvement of Platelet-Derived Microparticles and Microparticles Forming Immune Complexes during Monocyte Activation in Patients with Systemic Lupus Erythematosus
Read this article at
Abstract
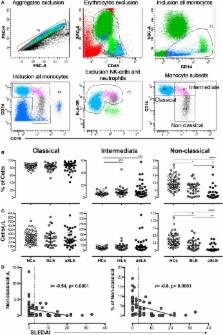
Microparticles (MPs) are vesicles derived from the plasma membrane of different cells, are considered a source of circulating autoantigens, and can form immune complexes (MPs-ICs). The number of MPs and MPs-ICs increases in patients with systemic lupus erythematosus (SLE). MPs activate myeloid cells by inducing IL-6 and TNF-α in both SLE and other diseases. Therefore, we propose that the recognition of MPs-ICs by monocytes rather that MPs may define their phenotype and contribute to the inflammatory process in patients with SLE. Thus, the aims of this study were to evaluate the association among circulating MPs-ICs from different cell sources, alterations observed in monocyte subsets, and disease activity in patients with SLE and to establish whether monocytes bind and respond to MPs-ICs in vitro. Circulating MPs and monocyte subsets were characterized in 60 patients with SLE and 60 healthy controls (HCs) using multiparametric flow cytometry. Patients had higher MP counts and frequencies of MPs-CD41a + (platelet-derived) compared with HCs, regardless of disease activity. MPs from patients with SLE were C1q + and formed ICs with IgM and IgG. MPs-IgG + were positively correlated with active SLE (aSLE), whereas MPs-IgM + were negatively correlated. Most of the circulating total ICs-IgG + were located on MPs. The proportion and number of non-classical monocytes were significantly decreased in patients with SLE compared with HCs and in patients with aSLE compared with patients with the inactive disease. Non-classical monocytes obtained from patients with SLE exhibited increased levels of CD64 associated with MPs-IgG +, MPs-C1q +, total circulating ICs-IgG +, and disease activity. The direct effects of MPs and MPs-IgG + on monocytes were evaluated in cell culture. Monocytes from both HCs and patients bound to and internalized MPs and MPs-IgG + independent of CD64. These vesicles derived from platelets (PMPs), mainly PMPs-IgG +, activated monocytes in vitro and increased the expression of CD69, CD64, and pro-inflammatory cytokines such as IL-1β, TNF-α, and IFN-α. Therefore, MPs are one of the most representative sources of the total amount of circulating ICs-IgG + in patients with SLE. MPs-IgG + are associated with SLE activity, and PMPs-IgG + stimulate monocytes, changing their phenotype and promoting pro-inflammatory responses related to disease activity.
Related collections
Most cited references61
- Record: found
- Abstract: found
- Article: not found
Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4.
- Record: found
- Abstract: found
- Article: not found
Type I interferon in systemic lupus erythematosus and other autoimmune diseases.
- Record: found
- Abstract: found
- Article: not found