- Record: found
- Abstract: found
- Article: not found
Heart failure-associated anemia: bone marrow dysfunction and response to erythropoietin
Read this article at
Abstract
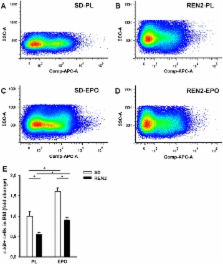
Heart failure (HF)-associated anemia is common and has a poor outcome. Because bone marrow (BM) dysfunction may contribute to HF-associated anemia, we first investigated mechanisms of BM dysfunction in an established model of HF, the transgenic REN2 rat, which is characterized by severe hypertrophy and ventricular dilatation and SD rats as controls. Secondly, we investigated whether stimulation of hematopoiesis with erythropoietin (EPO) could restore anemia and BM dysfunction. After sacrifice, erythropoietic precursors (BFU-E) were isolated from the BM and cultured for 10 days. BFU-E were quantified and transcript abundance of genes involved in erythropoiesis were assayed. Number of BFU-E were severely decreased in BM of REN2 rats compared to SD rats (50 ± 6.2 vs. 6.4 ± 1.7, p < 0.01). EPO treatment increased hematocrit in the SD-EPO group (after 6 weeks, 49 ± 1 vs. 58 ± 1%, p < 0.01); however, in the mildly anemic REN2 rats, there was no effect (43 ± 1 vs. 44 ± 1%). This was paralleled by a 67% decrease in BFU-E in BM of REN2 rats compared to SD ( p < 0.01). EPO significantly improved BFU-E in both SD and REN2 but could not restore this to control levels in the REN2 rats. Expression of several genes involved in differentiation (LMO2), mobilization (SDF-1), and iron incorporation (transferrin receptor) of the BM were differentially expressed in REN2 rats compared to SD rats, and EPO did not normalize this. Altogether, these results suggest that BM dysfunction is an important contributor to HF-associated anemia and that EPO is not an effective agent to treat HF-associated anemia.
Related collections
Most cited references29
- Record: found
- Abstract: found
- Article: not found
Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats.
- Record: found
- Abstract: found
- Article: not found
Anemia and mortality in heart failure patients a systematic review and meta-analysis.
- Record: found
- Abstract: found
- Article: not found
