- Record: found
- Abstract: found
- Article: found
A standardized and automated method of perineuronal net analysis using Wisteria floribunda agglutinin staining intensity
Read this article at
Abstract
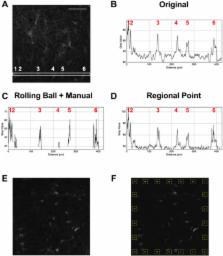
Perineuronal nets (PNNs) are aggregations of extracellular matrix molecules that are critical for plasticity. Their altered development or changes during adulthood appear to contribute to a wide range of diseases/disorders of the brain. An increasing number of studies examining the contribution of PNN to various behaviors and types of plasticity have analyzed the fluorescence intensity of Wisteria floribunda agglutinin (WFA) as an indirect measure of the maturity of PNNs, with brighter WFA staining corresponding to a more mature PNN and dim WFA staining corresponding to an immature PNN. However, a clearly-defined and unified method for assessing the intensity of PNNs is critical to allow us to make comparisons across studies and to advance our understanding of how PNN plasticity contributes to normal brain function and brain disease states. Here we examined methods of PNN intensity quantification and demonstrate that creating a region of interest around each PNN and subtracting appropriate background is a viable method for PNN intensity quantification that can be automated. This method produces less variability and bias across experiments compared to other published analyses, and this method increases reproducibility and reliability of PNN intensity measures, which is critical for comparisons across studies in this emerging field.
Highlights
Related collections
Most cited references27
- Record: found
- Abstract: found
- Article: not found
Perineuronal nets protect fear memories from erasure.
- Record: found
- Abstract: found
- Article: not found